| Identification | More | [Name]
ethyl 2-(4-hydroxyphenyl)-4-methyl thiazole-5-carboxylate | [CAS]
161797-99-5 | [Synonyms]
ethyl 2-(4-hydroxyphenyl)-4-methyl thiazole-5-carboxylate
Ethyl 2-(4-hydroxyphenyl)-4-methyl-1,3-thiazole-5-carboxylate | [EINECS(EC#)]
605-268-8 | [Molecular Formula]
C13H13NO3S | [MDL Number]
MFCD03700424 | [Molecular Weight]
263.31 | [MOL File]
161797-99-5.mol |
| Chemical Properties | Back Directory | [Melting point ]
180 ºC | [Boiling point ]
426.8±55.0 °C(Predicted) | [density ]
1.274 | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
DMSO (Slightly), Methanol (Very Slightly) | [form ]
Solid | [pka]
8.56±0.15(Predicted) | [color ]
Off-White | [InChI]
InChI=1S/C13H13NO3S/c1-3-17-13(16)11-8(2)14-12(18-11)9-4-6-10(15)7-5-9/h4-7,15H,3H2,1-2H3 | [InChIKey]
LOCYSKNNFCGDTR-UHFFFAOYSA-N | [SMILES]
S1C(C(OCC)=O)=C(C)N=C1C1=CC=C(O)C=C1 | [CAS DataBase Reference]
161797-99-5(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Uses]
Ethyl 2-(4-Hydroxyphenyl)-4-methylthiazole-5-carboxylate is used in the synthesis of the major metabolites of Febuxostat (F229000), a xanthine oxidase/xanthine dehydrogenase inhibitor. Used for treatment of hyperuricemia and chronic gout. 40-120 mg/day febuxostat was proven effective in lowering serum urate levels when administered to manage hyperuricemia in patients with gout. | [Synthesis]
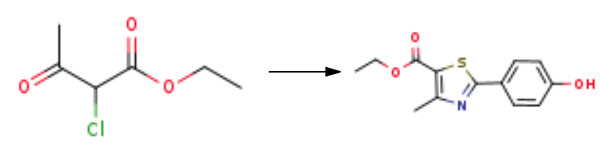
A mixture of 4-cyanophenol, NaOH, and ethanol was mixed in a pressure bottle while heated to 80° C. Hydrogen sulfide gas was then introduced, and the pressure increased to 30-60 psi until the thioamidation was determined to be complete by HPLC. Without isolating the thioamide product, HCl was added to the bottle until the pH was below 3.5, the H2S gas was removed, and the bottle was placed under a vacuum for 20 minutes at 30-40° C. The reaction was then heated to 70° C, and ethyl 2-chloroacetoacetate(1.1 eq.) was added to the reaction solution. The reaction was mixed under reflux for 2-3 hours, treated with enough H2O to dissolve the NaCl salt in the reaction mixture, cooled to room temperature, treated with enough water to precipitate the product, and the solid was collected by filtration. The precipitate was washed with water and dried at 80° C. with nitrogen bleeding to provide Ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate.
|
|
|