| Identification | More | [Name]
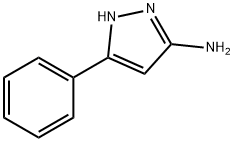
3-Amino-5-phenylpyrazole | [CAS]
1572-10-7 | [Synonyms]
3-AMINO-5-PHENYLPYRAZOLE
3-PHENYL-1H-PYRAZOL-5-AMINE
5-AMINO-3-PHENYLPYRAZOLE
5-PHENYL-1H-PYRAZOL-3-AMINE
5-PHENYL-1H-PYRAZOL-3-YLAMINE
5-PHENYL-2H-PYRAZOL-3-YLAMINE
AKOS 92617
AKOS B003768
BUTTPARK 30\09-86
LABOTEST-BB LT00080656
SALOR-INT L317837-1EA
TIMTEC-BB SBB005555
5-amino-3-phenyl-pyrazol
5-phenyl-1h-pyrazol-3-amin
3-Amino-5-phenyl-1H-pyrazole
5-Phenyl-1H-pyrazole-3-amine
3-Amino-5-phenyl-1H-pyrazole 97%
5-Phenyl-1H-pyrazole-3-ylamine
5-Amino-3-phenylpyrazole ,98% | [Molecular Formula]
C9H9N3 | [MDL Number]
MFCD00191749 | [Molecular Weight]
159.19 | [MOL File]
1572-10-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-white to cream colored crystalline powder | [Melting point ]
124-127 °C (lit.) | [Boiling point ]
442.3±33.0 °C(Predicted) | [density ]
1.238±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,Room temperature | [solubility ]
DMSO, Methanol | [form ]
Powder or Crystals | [pka]
14.80±0.10(Predicted) | [color ]
Red to brown | [Detection Methods]
HPLC | [BRN ]
4947 | [InChIKey]
PWSZRRFDVPMZGM-UHFFFAOYSA-N | [CAS DataBase Reference]
1572-10-7(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [RTECS ]
UQ6109000
| [Hazard Note ]
Irritant | [HS Code ]
29331990 | [Toxicity]
mouse,LDLo,intraperitoneal,630mg/kg (630mg/kg),Farmakologiya i Toksikologiya Vol. 27, Pg. 295, 1964. |
| Hazard Information | Back Directory | [Chemical Properties]
Off-white to cream colored crystalline powder | [Uses]
3-Amino-5-phenylpyrazole ((3-phenyl-1H-pyrazol-5-amine) may be used in the synthesis of the following:
- Urea derivatives by reaction with azido(6-(benzofuran-2-yl)-2-methylpyridin-3-yl) methanone.
- 2-Mercaptoacetamide analogs by treating with thioglycolic acid.
- 3-(Substituentpyrimidayl)-5,6-benzocoumarins by treating with 3-(2′-formyl-1′-chlorovinyl)-5,6-benzocoumarin.
- Substituted 2,7-diphenylpyrazolo[1,5-a]pyrimidine-5-carboxylic esters by reacting with substituted β-diketo esters.
- N-ethoxycarbonylthiourea derivative by reacting with ethoxycarbonyl isothiocyanate.
- Heterobiaryl pyrazolo[3,4-b]pyridines by reacting with indole-3-carboxaldehyde.
| [General Description]
3-Amino-5-phenylpyrazole (3-phenyl-1H-pyrazol-5-amine), an amino pyrazole derivative, is an aza-heterocyclic amine. It has been reported to be synthesized by heating either 3-amino-4-bromo- or 3-amino-5-phenylisothiazole in the presence of anhydrous hydrazine. On reaction with ZnCl2 it affords chlorido-tris(3-amino-5-phenyl-1Hpyrazole-N2)zinc (II) chloride. |
|
|