| Identification | More | [Name]
RUTHENIUM CARBONYL | [CAS]
15243-33-1 | [Synonyms]
DODECACARBONYLTRIRUTHENIUM
DODECACARBONYLTRIRUTHENIUM (0)
RUTHENIUM CARBONYL
TRIRUTHENIUM DODECACARBONYL
dodecacarbonyltri-rutheniutriangulo
Ru3(CO)12
Ruthenium, dodecacarbonyltri-, triangulo
Ruthenium,dodecacarbonyltri-
Rutheniumcarbonyl,99%
Ruthenium carbonyl 99%
Dodecacarbonyltriruthenium(0) Available in laboratory quantities only dec. 150
Ruthenium carbonyl, tri-Ruthenium dodecacarbonyl | [EINECS(EC#)]
239-287-4 | [Molecular Formula]
C12O12Ru3 | [MDL Number]
MFCD00011209 | [Molecular Weight]
639.33 | [MOL File]
15243-33-1.mol |
| Safety Data | Back Directory | [Hazard Codes ]
F,Xn | [Risk Statements ]
R11:Highly Flammable.
R20:Harmful by inhalation. | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
1325 | [WGK Germany ]
3
| [Hazard Note ]
Flammable/Harmful | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
II | [HS Code ]
28439000 |
| Hazard Information | Back Directory | [Description]
Triruthenium dodecacarbonyl is a metal carbonyl complex, classified as an inorganic compound. Its molecular formula is Ru3(CO)12, with a molecular weight of 639.33. It possesses D3h symmetry and a core composed of a Ru equilateral triangle. Each Ru atom is attached to two axial and two equatorial CO ligands. Unlike triiron dodecacarbonyl, this compound does not contain any bridge bonds. It is commonly employed as a precursor for various organoruthenium compounds. | [Chemical Properties]
orange plates | [Uses]
A H-transfer catalyst. It is used in carbonylation catalysis of the allylic amination of unactivated olefins by nitroarenes, reductive carbonylation of mononitro aromatic compounds to carbamates, and as a catalyst for cycloaddition reactions. It is employed as a precursor for the synthesis of Ru nanoparticles. Catalyst used in the intermolecular [3+2+1] cycloaddition of alpha,beta-unsaturated ketones with silylacetylenes and carbon monoxide, to produce alpha-pyrones. Catalyzes the coupling of silanes with thiols, alcohols and amines with high turnover number (TON) and high turnover frequency (TOF). | [Reactions]
-
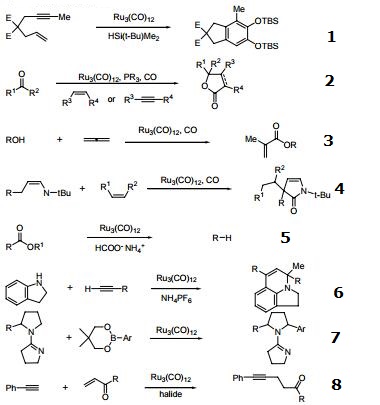
Catalyst for the conversion of enynes to catechol derivatives.
-
Catalyst for the intermolecular [2+2+1] cycloaddition of ketones, CO and alkenes or alkynes.
-
3-Component couplings.
-
Reaction of α,β-unsaturated imines with carbon monoxide and alkenes to form β,γ-unsaturated γ-butyroactams.
-
Ester decarboxylation.
-
Catalyst for hydroamination and C-H bond activation.
-
Used in sp3 C-H bond arylation and carbonylation.
-
Ru/halide catalytic system for C-C bond forming reaction between alkynes and unsaturated carbonyl compounds.
-
Amination of α-hydroxy amides.
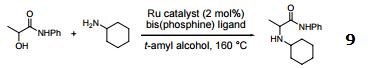
| [General Description]
Atomic number of base material: 44 Ruthenium | [reaction suitability]
core: ruthenium
reaction type: C-H Activation
reagent type: catalyst |
|
|