| Identification | More | [Name]
CYCLOPROPYL PHENYL SULFIDE | [CAS]
14633-54-6 | [Synonyms]
CYCLOPROPYL PHENYL SULFIDE
CYCLOPROPYL PHENYL SULPHIDE
(Cyclopropylsulfanyl)benzene
Phenylthiocyclopropane
Cyclopropyl phenyl sulfide, 98+%
Cyclopropylthiobenzene
1-(Phenylthio)cyclopropane | [Molecular Formula]
C9H10S | [MDL Number]
MFCD00009684 | [Molecular Weight]
150.24 | [MOL File]
14633-54-6.mol |
| Chemical Properties | Back Directory | [Appearance]
CLEAR YELLOWISH LIQUID | [Boiling point ]
62-63 °C/1 mmHg (lit.) | [density ]
1.045 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.581(lit.)
| [Fp ]
197 °F
| [form ]
clear liquid | [color ]
Colorless to Light yellow to Light orange | [Detection Methods]
GC,NMR | [BRN ]
2075701 | [CAS DataBase Reference]
14633-54-6(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [RIDADR ]
UN 2810 6.1/PG 3
| [WGK Germany ]
3
| [HazardClass ]
9 | [HS Code ]
29309090 |
| Hazard Information | Back Directory | [Chemical Properties]
CLEAR YELLOWISH LIQUID | [Uses]
Cyclopropyl phenyl sulfide was used as a reagent in the conversion of a ketone into a spirocyclobutanone. | [Synthesis Reference(s)]
Synthetic Communications, 10, p. 311, 1980 DOI: 10.1080/00397918008062755 | [Synthesis]
Phenylthiocyclopropane is easily prepared from 1-phenylthio-3-chloropropane (itself obtained on reaction of 1-bromo- 3-chloropropane and phenylthiolates) and Potassium Amide in liquid ammonia (eq 1).

1,3-Bis(phenylthio)propane reacts with n-Butyllithium in THF to produce Phenylthiocyclopropane on reaction with only 1 equiv n-Buli, or 1-lithio-1-phenylthiocyclopropane (2) if 2 equiv are used (eq 2).
Phenylthiocyclopropane has also been synthesized from 3-tributylstannylpropanal (available from tributylstannyllithium and Acrolein) and trimethylsilyl phenyl sulfide in the presence of Titanium(IV) Chloride as acid catalyst (eq 3), and on substitution of (i) Cyclopropyllithium with Diphenyl Disulfide (eq 4), (ii) cyclopropyl bromide with potassium benzenethiolate (eq 5) or (iii) 1,1-dibromocyclopropane, also with benzenethiolates, and by reduction of α- bromocyclopropyl phenyl sulfide with sodium methanethiolate (eq 6). In addition, cyclopropyl phenyl sulfoxide can be reduced to (1) in more than 70% yield with Chlorotrimethylstannane-benzenethiol (eq 7).
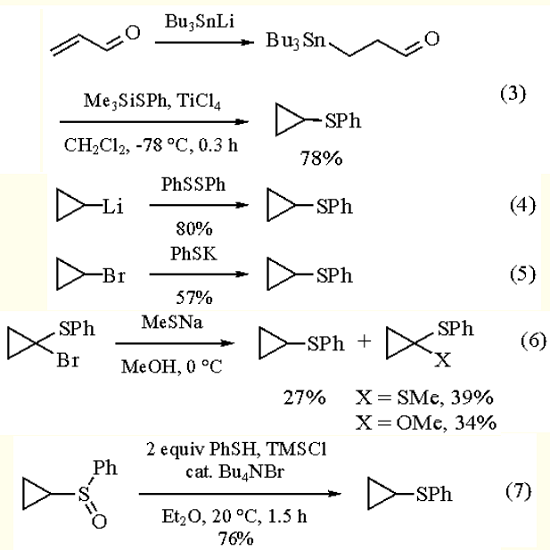 |
|
|