| Identification | Back Directory | [Name]
ACP196 | [CAS]
1420477-60-6 | [Synonyms]
ACP196
ACP-196
CS-1558
Calquence
EOS-60753
acalabrutinib
α-Dimethylglycine
ACP196,Acalabrutinib
ACP196 1420477-60-6
α-Aminoisobutanoic acid
Acalabrutinib (ACP-196)
ACP-196; ACP196; ACP 196
BTK Inhibitor Pharma Raw Powder Acalabrutinib / ACP-196 For Treatment of Cancer
4-[8-amino-3-[(2S)-1-but-2-ynoylpyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl]-N-pyridin-2-ylbenzamide
(S)-4-(8-amino-3-(1-but-2-ynoylpyrrolidin-2-yl)imidazo[1,5-a]pyrazin-1-yl)-N-(pyridin-2-yl)benzamide
4-[8-Amino-3-[(2S)-1-(1-oxo-2-butyn-1-yl)-2-pyrrolidinyl]imidazo[1,5-a]pyrazin-1-yl]-N-2-pyridinylbenzamide
Benzamide, 4-[8-amino-3-[(2S)-1-(1-oxo-2-butyn-1-yl)-2-pyrrolidinyl]imidazo[1,5-a]pyrazin-1-yl]-N-2-pyridinyl- | [EINECS(EC#)]
814-272-0 | [Molecular Formula]
C26H23N7O2 | [MDL Number]
MFCD29472294 | [MOL File]
1420477-60-6.mol | [Molecular Weight]
465.51 |
| Chemical Properties | Back Directory | [Melting point ]
>133°C (dec.) | [density ]
1.37±0.1 g/cm3(Predicted) | [storage temp. ]
Refrigerator | [solubility ]
Soluble in DMSO (up to at least 25 mg/ml) | [form ]
solid | [pka]
11.47±0.70(Predicted) | [color ]
Yellow | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. | [InChIKey]
WDENQIQQYWYTPO-IBGZPJMESA-N | [SMILES]
C(NC1=NC=CC=C1)(=O)C1=CC=C(C2=C3N(C([C@@H]4CCCN4C(=O)C#CC)=N2)C=CN=C3N)C=C1 |
| Hazard Information | Back Directory | [Description]
Acalabrutinib (ACP-196) is a selective second-generation Bruton's tyrosine kinase (BTK) inhibitor with an IC50 of 3 nM, which prevents the activation of the B-cell antigen receptor (BCR) signaling pathway. ACP-196 has improved target specificity over ibrutinib with 323-, 94-, 19- and 9-fold selectivity over the other TEC kinase family members (ITK, TXK, BMX, and TEC, respectively) and no activity against EGFR. | [Uses]
Acalabrutinib, is an experimental anti-cancer drug and a selective Bruton's tyrosine kinase (BTK) inhibitor. This kinase transmits signals from B-cell Receptor (BCR), and thus any genetic BTK mutation causes B-Cell immunodeficiency. Therefore, BTK inhibitors targeting B-cell signaling has shown great promise for the treatment of chronic lymphocytic leukemia (CLL). | [Definition]
ChEBI: Acalabrutinib is a member of the class of imidazopyrazines that is imidazo[1,5-a]pyrazine substituted by 4-(pyridin-2-ylcarbamoyl)phenyl, (2S)-1-(but-2-ynoyl)pyrrolidin-2-yl, and amino groups at positions 1, 3 and 8, respectively. It is an irreversible second-generation Bruton's tyrosine kinase (BTK) inhibitor that is approved by the FDA for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy. It has a role as an EC 2.7.10.2 (non-specific protein-tyrosine kinase) inhibitor, an antineoplastic agent and an apoptosis inducer. It is a secondary carboxamide, a member of benzamides, a member of pyridines, an aromatic amine, a pyrrolidinecarboxamide, an imidazopyrazine, a ynone and a tertiary carboxamide. | [General Description]
Class: non-receptor tyrosine kinase
Treatment: CLL, SLL, MCL
Oral bioavailability = 25%
Elimination half-life = 0.9 h
Protein binding = 97.5% | [in vitro]
In the in vitro signaling assay on primary human CLL cells, acalabrutinib inhibits tyrosine phosphorylation of downstream targets of ERK, IKB, and AKT. Acalabrutinib demonstrates higher selectivity for BTK with IC50 determinations on nine kinases with a cysteine residue in the same position as BTK. Importantly, unlike ibrutinib, acalabrutinib does not inhibit EGFR, ITK, or TEC. acalabrutinib has no effect on EGFR phosphorylation on tyrosine residues Y1068 and Y1173. Compared with ibrutinib, acalabrutinib has much higher IC50(>1000 nM) or virtually no inhibition on kinase activities of ITK, EGFR, ERBB2, ERBB4, JAK3, BLK, FGR, FYN, HCK, LCK, LYN, SRC, and YES1. | [in vivo]
Oral administration of ACP-196 in mice results in dose-dependent inhibition of anti-IgM-induced CD86 expression in CD19+ splenocytes with an ED50 of 0.34 mg/kg compared to 0.91 mg/kg for ibrutinib. A similar model is used to compare the duration of Btk inhibition after a single oral dose of 25 mg/kg. ACP-196 inhibits CD86 expression >90% at 3h postdose. | [IC 50]
3 nm | [Metabolism]
Acalabrutinib has an absolute bioavailability of 25% and an elimination half-life of 0.9 h. Because of its better oral bioavailability but much shorter half-life than ibrutinib, acalabrutinib is taken at a lower dosage (100 mg) than ibrutinib (420 mg) but twice a day (vs. QD for ibrutinib).
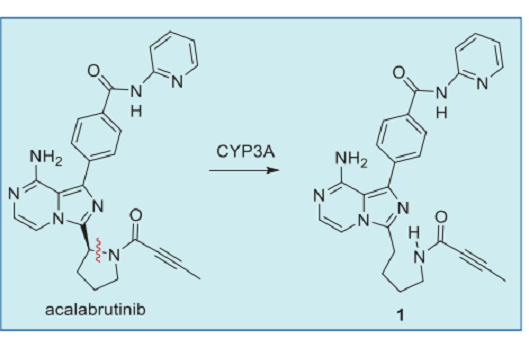
The major circulating metabolite in the plasma is the pyrrolidine ring-opened product 1. This metabolite also exhibited covalent BTK inhibition with potency 2-fold lower than acalabrutinib, and its kinase selectivity profile is similar to acalabrutinib. This active metabolite has a half-life of 6.9 h, much longer than the parent drug. However, the extent of its contribution to on-target covalent inhibition of BTK in humans remains to be established.
| [storage]
Store at -20°C | [References]
1) Wu et al.?(2016),?Acalabrutinib (ACP-196): a second-generation BTK inhibitor;?J. Hematol. Oncol.?9?21
2) Barf?et al.?(2017),?Acalabrutinib (ACP-196): A covalent Bruton Tyrosine Kinase Inhibitor with a Differentiated Selectivity and In Vivo Potency Profile;?J. Pharmacol. Exp. Ther.?363?240
3) Herman?et al.?(2017),?The Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia;?Clin. Cancer Res.?23?2831
4) Weber et al.?(2017),?Bruton’s Tyrosine Kinase: An Emerging Key Player in Innate Immunity; Front. Immunol.?8?1454 |
|
|