| Identification | Back Directory | [Name]
PACOCF3 | [CAS]
141022-99-3 | [Synonyms]
PTK
CAKB
FAK2
PTK2B
FADK2
RAFTK
CADTK
PACOCF3
PACOCF?
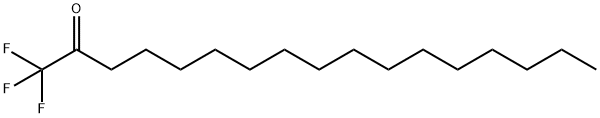
1,1,1-trifluoroheptadecan-2-one
1,1,1-TRIFLUORO-2-HEPTADECANONE
PALMITYL TRIFLUOROMETHYL KETONE
PALMITOYL TRIFLUOROMETHYL KETONE
1,1,1-TRIFLUOROHEPTADECANE-2-ONE
2-Heptadecanone, 1,1,1-trifluoro-
Pentadecyl trifluoromethyl ketone
PACOCF - CAS 141022-99-3 - Calbiochem
PYK2 (360-690), active, His tagged human
Palmityl trifluoromethyl ketone phosholipase A2 inhibitor | [Molecular Formula]
C17H31F3O | [MDL Number]
MFCD00797669 | [MOL File]
141022-99-3.mol | [Molecular Weight]
308.42 |
| Chemical Properties | Back Directory | [Boiling point ]
329.0±37.0 °C(Predicted) | [density ]
0.955±0.06 g/cm3(Predicted) | [RTECS ]
MI3927000 | [Fp ]
14℃ | [storage temp. ]
−20°C | [solubility ]
Soluble in DMSO | [form ]
Liquid | [color ]
White to off-white | [biological source]
synthetic (organic) | [Specific Activity]
216-292nmol/min·mg |
| Hazard Information | Back Directory | [Description]
PTK is an analog of palmitic acid in which the COOH group is replaced by trifluoromethyl ketone. In P388D1 cells, PTK inhibits iPLA2 activity with an IC50 value of 3.8 μM. A related compound, ATK is a less potent inhibitor of iPLA2 with an IC50 value of 15 μM. PTK also inhibits the activity of cPLA2 with an IC50 value almost identical to that of ATK. | [Uses]
PACOCF3 is an inductors of BLM, an artificial lipid membranes and liposome permeabilization. Phospholipase A2 inhibitor. | [Definition]
ChEBI: Palmityl Trifluoromethyl Ketone is an alpha-haloketone. | [Biological Activity]
Phospholipase A 2 inhibitor. Can also alter Ca 2+ signaling in renal tubular cells. | [References]
1. murakami m et al.emerging roles of secreted phospholipase a2 enzymes: the 3rd edition.biochimie. 2014 sep 16. pii: s0300-9084(14)00252-1.2. quach nd et al. secretory phospholipase a2 enzymes as pharmacological targets for treatment of disease. biochem pharmacol. 2014 aug 15;90(4):338-48. 3. chalimoniuk m. secretory phospholipase a2 and its role in oxidative stress and inflammation]. postepy biochem. 2012;58(2):204-8.4. persaud sj.et al. the role of arachidonic acid and its metabolites in insulin secretion from human islets of langerhans. diabetes. 2007 jan;56(1):197-203.5. khakpour h et al. lipoprotein-associated phospholipase a2: an independent predictor of cardiovascular risk and a novel target for immunomodulation therapy. cardiol rev. 2009 sep-oct;17(5):222-9. 6. narendra sharath chandra jn1 et al. chemistry and structural evaluation of different phospholipase a2 inhibitors in arachidonic acid pathway mediated inflammation and snake venom toxicity. curr top med chem. 2007;7(8):787-800.7. packard cj. lipoprotein-associated phospholipase a2 as a biomarker of coronary heart disease and a therapeutic target. curr opin cardiol. 2009 jul;24(4):358-63.8. lucas r et al. synthesis and enzyme inhibitory activities of a series of lipidic diamine and aminoalcohol derivatives on cytosolic and secretory phospholipases a2. bioorg med chem lett. 2000 feb 7;10(3):285-8. |
|
| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
|
| Tel: |
|
| Website: |
www.sangon.com |
|