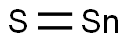| Identification | More | [Name]
TIN (II) SULFIDE | [CAS]
1314-95-0 | [Synonyms]
STANNOUS SULFIDE
TIN (II) SULFIDE
TIN MONOSULFIDE
TIN SULFIDE
SnS
Tin sulfide (SnS)
tinsulfide(sns)
tin sulphide
Tin(Ⅳ) sulfide
tin disulfide
Tin protosulfide
TIN(II)SULPHIDE
Tin(II) sulfide, 99.5% | [EINECS(EC#)]
215-248-7 | [Molecular Formula]
SSn | [MDL Number]
MFCD00011245 | [Molecular Weight]
150.77 | [MOL File]
1314-95-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Dark-gray or black, crystalline powder. Soluble in concentrated
hydrochloric acid (decomposes); insoluble in
dilute acids and water. | [Melting point ]
882°C | [Boiling point ]
1202.34°C (estimate) | [density ]
5.22 g/mL at 25 °C(lit.)
| [solubility ]
insoluble in H2O; soluble in concentrated acid solutions | [form ]
-8 Mesh | [Specific Gravity]
5.08 | [Water Solubility ]
Soluble in conc. HCl, hot conc. H{2}SO{4}.Insoluble in water. | [crystal system]
Nogata | [Merck ]
14,8791 | [Solubility Product Constant (Ksp)]
pKsp: 25.00 | [Space group]
Pmcn | [Lattice constant]
| a/nm | b/nm | c/nm | α/o | β/o | γ/o | V/nm3 | | 0.4334 | 1.12 | 0.3987 | 90 | 90 | 90 | 0.1935 |
| [Exposure limits]
ACGIH: TWA 2 mg/m3
NIOSH: IDLH 100 mg/m3; TWA 2 mg/m3 | [LogP]
-2.47 | [CAS DataBase Reference]
1314-95-0(CAS DataBase Reference) | [EPA Substance Registry System]
Tin sulfide (SnS) (1314-95-0) | [Application]
In the form of single or few-layer thin films,?exfoliated SnS?nanosheets have various applications.?These include light emitters, field effect transistors (FETs), gas sensors, photodetectors, thermoelectric and photovoltaic devices. | [Bandgap]
1.07 -1.32 eV | [Chemical Synthesis]
Tin sulfide?(SnS)?is manufactured using chemical vapour transport (CVT) crystallisation, with crystals having a purity in excess of 99.999%. | [color ]
Brown/Yellow | [Description]
Tin sulfide (SnS), with a direct energy band-gap of about 1.3 eV, and a high optical absorption coefficient over 5 × 104 cm-1,?is a promising new candidate for applications in the next generation of photovoltaic solar cells. Made of earth-abundant, relatively cheap and environmentally-nontoxic elements, SnS is solution processable and stable in both alkaline and acidic conditions. | [Electronic properties]
2D semiconductor | [Preparation]
Synthetic?- Chemical Vapour Transport (CVT) | [Usage ]
Tin sulfide single crystals?can be used to prepare monolayer and few-layer SnS?by mechanical or liquid exfoliation. |
| Hazard Information | Back Directory | [Synthesis]
Tin sulfide?(SnS)?is manufactured using chemical vapour transport (CVT) crystallisation, with crystals having a purity in excess of 99.999%. | [Chemical Properties]
Dark-gray or black, crystalline powder. Soluble in concentrated
hydrochloric acid (decomposes); insoluble in
dilute acids and water. | [Uses]
Polymerization catalyst. | [Uses]
The p-type IV-VI semiconductor tin(II) sulfide is of interest for use in optoelectronic devices. SnS is also used as a nontoxic, inexpensive component in heterojunction photovoltaic devices. | [Hazard]
Toxic material. | [Flammability and Explosibility]
Notclassified |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [TSCA ]
Yes |
|
|