| Identification | More | [Name]
Digold trioxide | [CAS]
1303-58-8 | [Synonyms]
digold trioxide
GOLD(III) OXIDE
GOLD OXIDE
GOLD SESQUIOXIDE
goldoxide(au2o3)
Goldoxide,trihydrate
Auric oxide
Gold(III) oxide hydrate
GoldoxideorangebrownpowderGoldgehalt
Gold(III) oxide, 99.9%
Gold trioxide
Gold(III)oxide,99%
gold(iii) oxide, premion
Auric hydroxide
Gold(III) Oxide, Au 89%,
Gold(III) oxide, Premion(R), 99.99% (metals basis), Au 88.6% min
Gold (III) Oxide, Au 89%
Gold(III) oxide, Premion, 99.99% (metals basis), Au 88.6% min | [EINECS(EC#)]
215-122-1 | [Molecular Formula]
Au2O3 | [MDL Number]
MFCD00014173 | [Molecular Weight]
441.93 | [MOL File]
1303-58-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Brownish-black powder, decomposed
by heat.Keep in dark bottle. Soluble in hydrochloric
acid; insoluble in water. | [Melting point ]
150°C | [solubility ]
insoluble in H2O; soluble in acid solutions | [form ]
Powder | [color ]
Brown | [Water Solubility ]
Insoluble in water. Soluble in HCl, HNO<sub>3</sub> and in NaCN solution. | [Merck ]
14,4524 | [Uses]
Gold plating | [CAS DataBase Reference]
1303-58-8(CAS DataBase Reference) | [EPA Substance Registry System]
1303-58-8(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/38:Irritating to eyes and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [RIDADR ]
UN 3263 8/PG 3
| [WGK Germany ]
3
| [TSCA ]
Yes | [HS Code ]
2843300000 |
| Questions And Answer | Back Directory | [General description]
Brown powder; decomposes slowly on exposure to sunlight or by heating at 150°C; begins to release oxygen at 110°C; fully decomposes to metallic gold at 250°C; insoluble in water; soluble in hydrochloric and concentrated nitric acids; also soluble in aqueous solutions of sodiumor potassium cyanide.
| [Preparation]
Gold(III) oxide is prepared by heating gold(III) hydroxide, Au(OH)3 at 130 to 140°C:
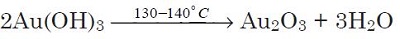
|
| Hazard Information | Back Directory | [Chemical Properties]
Brownish-black powder, decomposed
by heat.Keep in dark bottle. Soluble in hydrochloric
acid; insoluble in water. | [Purification Methods]
The most probable impurities are SO4and Cl-ions. Dissolve it in strong boiling KOH solution (ca 5M) and precipitate (care) with excess of 3N H2SO4. Then shake and centrifuge, resuspend in H2O and repeat the washing several times until free from SO4 and Cl ions. This gives a wet oxide which is dried in air, but decomposes to free gold in sunlight. It is advisable to keep it wet as it decomposes on drying (analyse wet sample). Store it away from light in the presence of H2O vapour. It evolves O2 at 110o. It is insoluble in H2O but soluble in HCl and conc HNO3. [Roseveare & Buehrer J Am Chem Soc 49 1221 1927.] |
|
|