| Identification | More | [Name]
Fulvestrant | [CAS]
129453-61-8 | [Synonyms]
(7a,17b)-7-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol
7A,17B-[9[(4,4,5,5,5-PENTAFLUOROPENTYL)SULFINYL]NONYL]ESTRA-1,3,5(10)-TRIENE-3,17-DIOL
FASLODEX
FULVESTRANT
FULVESTRANT-13C1-D2
ICI 182,780
(7alpha,17beta)-nonyl)
estra-1,3,5(10)-triene-3,17-diol,7-(9-((4,4,5,5,5-pentafluoropentyl)sulfinyl)
zd182780
zm182780
(717b)-�7-[9-[4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol
ICH-182780
7α-[9-[(4,4,5,5,5,-Pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17β-diol
ICI-187280
Fulvstrant
(7,17)-7-[9-[4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol
Faslodex, ICI 182,780, ZD 182780, ZD 9238, ZM 182780, (7a,17b)-7-[9-[(4,4,5,5,5pentafluoropentyl)Sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol | [EINECS(EC#)]
642-998-6 | [Molecular Formula]
C32H47F5O3S | [MDL Number]
MFCD00903953 | [Molecular Weight]
606.77 | [MOL File]
129453-61-8.mol |
| Chemical Properties | Back Directory | [Appearance]
White Powder | [Melting point ]
104-106°C | [Boiling point ]
674.8±55.0 °C(Predicted) | [density ]
1.201±0.06 g/cm3(Predicted) | [storage temp. ]
2-8°C | [solubility ]
DMSO: >5mg/mL | [form ]
powder | [pka]
10.27±0.70(Predicted) | [color ]
White | [Usage]
Antineoplastic (hormonal) | [Stability:]
Stable for 2 years as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. | [InChIKey]
VWUXBMIQPBEWFH-WCCTWKNTSA-N | [SMILES]
[C@@]12([H])[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)CC3C=C(O)C=CC=3[C@@]1([H])CC[C@]1(C)[C@H](CC[C@@]21[H])O |&1:0,2,32,36,38,41,r| | [CAS DataBase Reference]
129453-61-8(CAS DataBase Reference) |
| Questions And Answer | Back Directory | [Indications and Usage]
Fulvestrant is a muscle injection drug developed by the company AstraZeneca and is suitable for treating postmenopausal women with estrogen receptor-positive metastasized breast cancer whose condition continued to worsen despite antiestrogen treatment. Fulvestrant is the only antiestrogen drug that can be widely clinically used following unsuccessful tamoxifen treatment. This drug is a type of endocrine therapy, so it will not cause any adverse effects commonly seen in chemotherapy, giving it relatively good patient compliance. Multiple clinical trials have found that 250mg Fulvestrant is effective and consistently safe as a second line of treatment for advanced breast cancer.
| [Mechanisms of Action]
Many breast cancer cells contain estrogen receptors (ER), so estrogen stimulates breast cancer growth. Fulvestrant is a steroid estrogen receptor antagonist, and its chemical structure is similar to estradiol, except that its 7α position contains a linking group. Fulvestrant is a 17β-estradiol alkylamine analogue, and it binds with, prevents, and decreases ER to inhibit the estrogen signal transduction pathway. It binds competitively with ER, has a similar affinity with ER as estrogen, and inhibits gene activation stimulated by estrogen, thus affecting necessary estrogen-related processes in cell circulation. Its fulvastans have a similar affinity with ER as estrogen and is 100 times that of tamoxifen.
| [Pharmacokinetics]
Fulvestrant has a relatively poor oral bioavailability, so it is commonly injected into the muscle with lipids as excipients. In an open, random and multicenter study on postmenopausal women with advanced breast cancer, one 5ml or 2 2.5ml dosages containing 250mg were injected, and their pharmacokinetics and poisonous side effects did not differ greatly, while its blood concentration was dose-dependent and had individual differences. In the 7-day treatment period, serum LH, FSH or SBHG levels did not change significantly. This drug does not pass through the blood-brain barrier and will not cause side effects such as vasomotor symptoms.
| [Adverse reactions]
Fulvestrant causes relatively fewer side effects, including brief vaginal bleeding, body odor change, and sleepwalking. There have not been any reports of effects such as vaginal dryness, weight gain, blood clotting abnormalities, thrombus formation and libido change, and characteristics such as facial flushing and sweating are not affected. A small-scale stage III clinical trial on 19 women with metastasized breast cancer who used this drug showed that its clinical efficacy was 67% and there were no serious safety issues. It showed that continuous monthly injections were crucial and that it was well-tolerated, with only slight swelling and paint at injection site, while facial flushing, uterine lining thickness, sex hormone binding globulin levels, follicle stimulating hormones levels, and luteinizing hormone levels all showed no change.
|
| Hazard Information | Back Directory | [Description]
Fulvestrant was launched in the US as a novel once monthly injectable steroidal
estrogen antagonist for the treatment of hormone receptor positive metastatic breast
cancer in postmenopausal women with disease progression following estrogen therapy.
This 7a-alkylsulphinyl derivative of estradiol can be prepared in 10 steps from 6,7-
didehydro-19-nor-testosterone by successive conjugate addition of the organocuprate
derived from O-protected 9-bromononan-l-o1 followed by aromatization of the resulting
enone, then activation of the protected primary alcohol, substitution with 4,4,5,5,5-
pentafluoropentanthiol and oxidation to the sulfoxide. Fulvestrant is the first “pure”
estrogen antagonist from a novel class known as selective estrogen receptor down
regulators (SERDs). It binds to the estrogen receptor (ER), with affinity close to that of
estradiol and 100 fold greater than that of tamoxifen (a partial estrogen antagonist),
preventing estrogen-stimulated gene activation, thereby interfering with the estrogenrelated
processes essential for cell-cycle completion. Fulvestrant also appears to
downregulate the ER by 80-90% often to non detectable level both in vitro and in vivo. In
comparison to tamoxifen, fulvestrant is devoid of systemic estrogenic activity, it displays no
uterotrophic activity and is able to block the uterine stimulation of estradiol or tamoxifen.
Furthermore, fulvestrant completely blocks the cell growth in tamoxifen-resistant breast
cancer cell-lines and prevents growth of tamoxifen resistant tumor in mice. In clinical trials,
it was also shown that fulvestrant is comparable to anastrozole (a third generation
aromatase inhibitor) both in efficacy and tolerability in postmenopausal women with
tamoxifen-resistant advanced breast cancers. | [Chemical Properties]
White or almost white powder. | [Originator]
Astra Zeneca (UK) | [Uses]
A novel steroidal estrogen antagonist reported to lack any partial agonist activity. Antineoplastic (hormonal). | [Uses]
antiestrogen | [Definition]
ChEBI: A 3-hydroxy steroid that is 17beta-estradiol in which the 7alpha hydrogen has been replaced by a nonyl group in which one of the hydrogens of the terminal methyl has been replaced by a (4,4,5,5,5-pentafluoropentyl)sulfinyl
group. An estrogen receptor antagonist, it is used in the treatment of breast cancer. | [Brand name]
Faslodex (AstraZeneca). | [General Description]
Fulvestrant, 7α-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17β-diol (Faslodex), is an antagonist structurally based onthe estradiol structure, with a long, substituted alkyl chainattached at the 7α-position of the steroid skeleton. Whenbound to the ERs, this alkyl chain induces a conformationof the receptor distinctive from that formed upon estradiolor tamoxifen binding, preventing agonist action.Fulvestrant is a pure antagonist at both ERαand ERβandan ER downregulator (stimulates degradation of the ER),completely lacking the agonist activity that is seen with tamoxifenor raloxifene. The different pharmacological profileof fulvestrant allows the use of this agent in womenwho have had disease progression after prior antiestrogentherapy (typically tamoxifen), providing an alternative toaromatase inhibitors. | [Biological Activity]
A high affinity estrogen receptor antagonist (IC 50 = 0.29 nM), devoid of any partial agonism both in vitro and in vivo . Also high affinity agonist at the membrane estrogen receptor GPR30. | [Biochem/physiol Actions]
Fulvestrant (ICI 182,780) is a selective estrogen receptor down-regulator (SERD). Fulvestrant is a high affinity estrogen receptor antagonist. IC50 = 0.29 nM. Fulvestrant is the first "pure" antiestrogen with no agonistic activity both in vitro and in vivo. | [Clinical Use]
Treatment of postmenopausal women with oestrogenreceptor-
positive, locally advanced or metastatic breast
cancer | [Side effects]
Side effects appear to be minimal and include several GI symptoms , headache, and hot flashes . There is no clinical evidence of uterine stimulation or laboratory evidence of stimulation of endometrial carcinoma models. Fulvestrant should not be adm inistered to women who are pregnant, who are taking antic oagulants, or who have thrombocytopenia. | [Synthesis]
Fulvestrant is administered as a once a month
i. m. injection. Several routes for the synthesis of fulvestrant
(12) were published. One of the best routes is
depicted in the scheme. The conjugate addition of Grignard
reagent derived from bromide 130 with dienone 129 gave
adduct 131 as a mixture of 7|á- and 7|?-isomers in a ratio of
2.5:1 in 90-95% yield. Aromatization of the A-ring with
copper bromide/lithium bromide in acetic acid followed by
hydrolysis of the ester group provided diol 132 in 80-85%
yield. Oxidation of the side chain from sulfite to sulfone
followed by crystallization provided fulvestrant (12) in 30%
overall yield from dienone 129.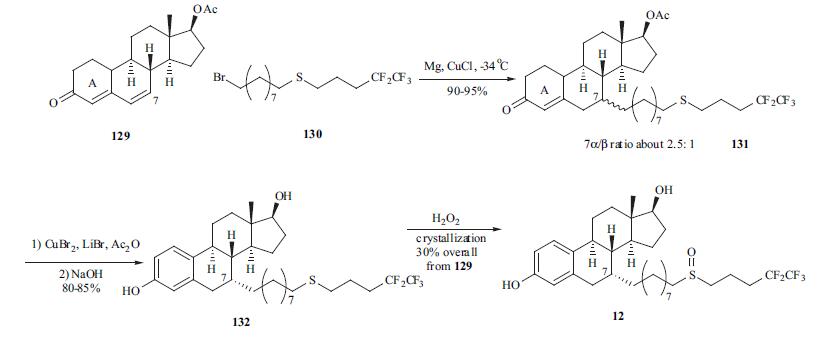
| [Drug interactions]
Potentially hazardous interactions with other drugs
None known | [Metabolism]
The metabolism of fulvestrant has not been fully
evaluated, but involves combinations of a number of
possible biotransformation pathways analogous to those
of endogenous steroids. Identified metabolites (includes
17-ketone, sulphone, 3-sulphate, 3- and 17-glucuronide
metabolites) are either less active or exhibit similar
activity to fulvestrant in anti-oestrogen models.
Fulvestrant is eliminated mainly in metabolised form. The
major route of excretion is via the faeces. | [storage]
Store at -20°C | [References]
Osborne et al. (2004), Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action; Br. J. Cancer 90 (Suppl 1):S2
Thomas et al. (2005), Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells; Endocrinology 146 624
Wardley (2002), Fulvestrant: a review of its development, pre-clinical and clinical data; Int. J. Clin. Pract. ?56 305
Castro et al. (2012),?Coumestrol has neuroprotective effects before and after cerebral ischemia in female rats; Brain Res.?1474 82
Blackburn et al. (2018),?Fulvestrant for the treatment of advanced breast cancer; Expert Rev. Anticancer Ther. 18 619 |
|
|