| Identification | More | [Name]
Fludrocortisone | [CAS]
127-31-1 | [Synonyms]
4-PREGNEN-9-ALPHA-FLUORO-11-BETA, 17,21-TRIOL-3,20-DIONE
4-PREGNEN-9ALPHA-FLUORO-11BETA, 17ALPHA, 21-TRIOL-3, 20-DIONE
4-PREGNENE-9-ALPHA-FLUORO-11-BETA,17-ALPHA,21-TRIOL-3,20-DIONE
9a-fluoro-11b,17a,21-trihydroxy-4-pregnene-3,20-dione
9a-fluoro-17-hydroxycorticosterone
9-ALPHA-FLUORO-11-BETA,17-ALPHA,21-TRIHYDROXY-4-PREGNENE-3,20-DIONE
9-ALPHA-FLUOROHYDROCORTISONE
9-FLUOROCORTISOL
FLUDROCORTISONE
FLUOROCORTISONE
17,21-trihydroxy-20-dion(11beta)-pregn-4-ene-9-fluoro-11
9alpha-fludrocortisone
9-alpha-fluoro-17-hydroxycorticosterone
9alpha-fluoro-17-hydroxycorticosterone
9-alpha-fluorocortisol
9alpha-fluorocortisol
9-fluoro-11-beta,17,21-trihydroxy-pregn-4-ene-20-dione
9-fluoro-11beta,17,21-trihydroxy-pregn-4-ene-20-dione
9-fluoro-11-beta,17,21-trihydroxypregn-4-ene-3,20-dione
9-fluorohydrocortisone | [EINECS(EC#)]
204-833-2 | [Molecular Formula]
C21H27FO5 | [MDL Number]
MFCD00083333 | [Molecular Weight]
378.43 | [MOL File]
127-31-1.mol |
| Chemical Properties | Back Directory | [Appearance]
White Solid | [Melting point ]
208-212°C | [alpha ]
D23 +139° (c = 0.55 in 95% ethanol) | [Boiling point ]
564.7±50.0 °C(Predicted) | [density ]
1.1176 (estimate) | [storage temp. ]
Refrigerator | [solubility ]
DMSO (Slightly), Ethanol (Slightly, Heated), Methanol (Slightly, Sonicated) | [form ]
Solid | [pka]
12.11±0.70(Predicted) | [color ]
White to Off-White | [Water Solubility ]
111mg/L(25 ºC) | [Usage]
A mineralocorticoid | [CAS DataBase Reference]
127-31-1(CAS DataBase Reference) | [EPA Substance Registry System]
127-31-1(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
White Solid | [Originator]
Alflorone Acetate,MSD,US,1954 | [Uses]
A mineralocorticoid | [Uses]
A mineralocorticoid. | [Definition]
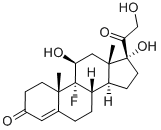
ChEBI: Fludrocortisone is a C21-steroid, a 3-oxo-Delta(4) steroid, a 20-oxo steroid, a 21-hydroxy steroid, a fluorinated steroid, a mineralocorticoid, a 17alpha-hydroxy steroid and an 11beta-hydroxy steroid. It has a role as an adrenergic agent and an anti-inflammatory drug. It derives from a hydride of a pregnane. | [Manufacturing Process]
Hydrocortisone acetate is first reacted with phosphorus oxychloride in pyridine
to give the corresponding olefin. Then a sequence consisting of hypobromous
acid addition, ring closure to the epoxide and ring opening with hydrogen
fluoride gives fludrocortisone acetate. Preparation of a crystalline product is
described then in US Patent 2,957,013. | [Brand name]
Florinef (King). | [Therapeutic Function]
9-Fluoro-11β,17,21-trihydroxy-pregn-4-ene-3,20-dione
acetate | [Clinical Use]
Fludrocortisone acetate is used
orally for mineralocorticoid replacement therapy in patients with adrenocortical insufficiency, such as Addison's
disease. This drug, introduced in 1954, helped to provide the impetus for the synthesis and biological
evaluation of newer halogenated analogues. | [Synthesis]
Fludrocortisone, 9|á-fluoro-11|?,17|á,21-trihydroxypregn-4-en-3,20-
dione (27.2.14), is synthesized from hydrocortisone acetate (27.1.17). In the first stage of
synthesis, dehydration of the hydrocortisone molecules is accomplished using phosphorous
chloride in pyridine, which forms a product with a double bond at C9¨CC11 27.2.11. The
resulting double bond is synthesized into an epoxide by an initial transformation to a bromohydrine
using N-bromoacetamide and subsequent dehydrobromination using sodium
acetate, which forms 21-O-acetoxy-9d-11|?-epoxy-17|á-hydroxy-4-pregnen-3,20-dione
(27.2.12). As described above, the epoxide ring is opened by hydrofluoric acid, which results
in the formation of the 21-O-acetate of fludrocortisone 27.2.13. Hydrolysis of the acetyl
group of this compound using potassium acetate gives fludrocortisone (27.2.14).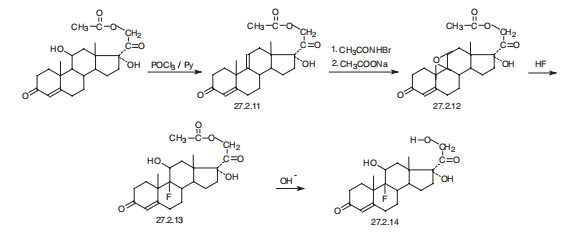
|
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
|