| Identification | Back Directory | [Name]
OXYPHENCYCLIMINE | [CAS]
125-52-0 | [Synonyms]
oximin
setrol
s1-1236
dominil
enterex
daricol
daricon
syklifen
vio-thene
ulcociclinina
tehydrochloride
OXYPHENCYCLIMINE
oxyphencyclimineHCl
Hydrobenzole Hydrochlordum
Hydrobenzole Hydrochloridum
OXYPHENCYCLIMINE HYDROCHLORIDE
OXYPHENCYCLIMINE HYDROCHLORIDE 98%
inyl)methylester,monohydrochloride
1-methyl-1,4,5,6-tetrahydro-2-pyrimidylmethyl-alpha-cyclohexyl-phenylglycola
cyclohexaneglycolicacid,alpha-phenyl-,(1,4,5,6-tetrahydro-1-methyl-2-pyrimid
Benzeneacetic acid, .alpha.-cyclohexyl-.alpha.-hydroxy-, (1,4,5,6-tetrahydro-1-methyl-2-pyrimidinyl)methyl ester, monohydrochloride | [EINECS(EC#)]
204-742-8 | [Molecular Formula]
C20H28N2O3.ClH | [MDL Number]
MFCD00079209 | [MOL File]
125-52-0.mol | [Molecular Weight]
380.91 |
| Hazard Information | Back Directory | [Originator]
Vio-Thene ,Rowell,US,1959 | [Uses]
Oxyphencyclimine is widely used for the same indications as dicyclomine and oxybutynin. | [Definition]
ChEBI: Oxyphencyclimine hydrochloride is a member of pyrimidines. | [Manufacturing Process]
To a stirred solution of 8.8 grams (0.1 mol) of 1,3-diaminobutane in 150 ml of ethanol maintained at 0° to 5°C, there was added 25.8 grams (0.1 mol) of ethyl chlorimidoacetate hydrochloride during a period of 20 minutes. After the mixture had been stirred at 0° to 5°C for two hours, it was acidified at this temperature by the addition of ethanolic hydrogen chloride. The mixture was warmed to room temperature and filtered to remove 4.3 grams of solid ammonium chloride. The filtrate was concentrated to approximately 40 ml, filtered and refrigerated. The solid which separated was isolated, washed with acetone and dried. There was obtained 7.4 grams (40% of the theoretical yield) of 2-chloromethyl-4-methyl-1,4,5,6-tetrahydropyrimidine hydrochloride melting at 158° to 160°C.
In a second step, cyclohexyl bromide was reacted with magnesium, then with benzoyl formic acid to give cyclohexylphenyl glycolic acid. A solution of 1.8 grams (0.01 mol) of 2-chloromethyl-1-methyl-1,4,5,6-tetrahydropyrimidine hydrochloride in 5 ml of water was made alkaline with 5 ml of 50% NaOH and extracted with ether. The ether solution, which contained the basic chloride, was dried over calcium sulfate and added to a solution of 2.3 grams (0.01 mol) of α-cyclohexylphenylglycolic acid in 75 ml of isopropanol. The solution was distilled to remove the ether, and 0.1 gram of powdered potassium iodide added to the residual isopropanol solution which was then refluxed for 6 hours. The solid which had separated was redissolved by the addition of 20 ml of ethanol and the solution charcoaled, concentrated, and cooled. The solid which separated, 1-methyl-1,4,5,6-tetrahydro-2-pyrimidylmethyl αcyclohexylphenyl-glycolate hydrochloride, weighed 1.4 grams and melted at 228° to 229°C with decomposition after recrystallization from ethanol.
| [Brand name]
Daricon (Pfizer). | [Therapeutic Function]
Spasmolytic | [General Description]
Oxyphencycliminehydrochloride, 1,4,5,6-tetrahydro-1-methyl-2-pyrimidinyl)methyl -phenylcyclohexaneglycolate monohydrochloride(Daricon, Vistrax), was introduced in 1958 and promoted asa peripheral anticholinergic–antisecretory agent, with little orno curare-like activity and little or no ganglionic blocking activity.These activities are probably absent because of the tertiarycharacter of the molecule. This activity is in contrastwith that of compounds that couple antimuscarinic actionwith ganglionic blocking action. The tertiary character of thenitrogen promotes intestinal absorption of the molecule.Perhaps the most significant activity of this compound is itsmarked ability to reduce both the volume and the acid contentof the gastric juices, a desirable action in view of the more recenthypotheses pertaining to peptic ulcer therapy. Anotherimportant feature of this compound is its low toxicity in comparisonwith many of the other available anticholinergics.Oxyphencyclimine hydrochloride is hydrolyzed in the presenceof excessive moisture and heat. It is absorbed from theGI tract and has a duration of action of up to 12 hours. | [Clinical Use]
Oxyphencyclimine hydrochloride is suggested for usein peptic ulcer, pylorospasm, and functional bowel syndrome.It is contraindicated, as are other anticholinergics,in patients with prostatic hypertrophy and glaucoma. | [Synthesis]
Oxyphencylimine, the 1,4,5,6-tetrahydro-1-methyl-2-pyrimidinyl�methanolic ester of |á-phenylcyclohexaneglycolic acid (14.1.37), is synthesized by the esterification of |á-phenyl-|á-cyclohexaneglycolic acid with 2-chloromethyl-1-methyl-
1,4,5,6-tetrahydropyrimidine (14.1.36) in the presence of potassium iodide. The initial
2-chloromethyl-1-methyl-1,4,5,6-tetrahydropyrimidine (14.1.36), is synthesized in turn by
reacting methyl ester of iminochloracetic acid with 3-methylaminopropylamine [27¨C29].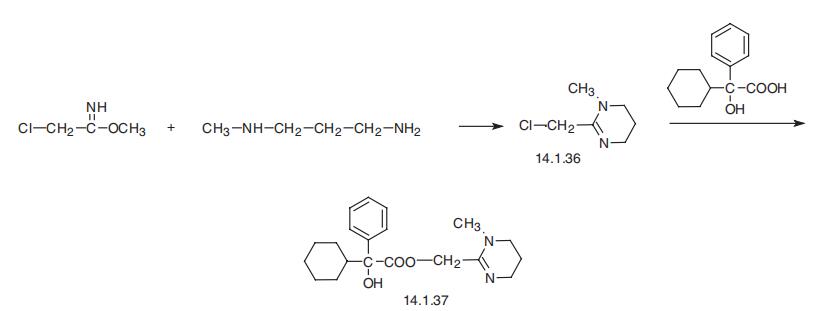
|
|
| Company Name: |
Alfa Chemistry
|
| Tel: |
1-516-6625404 |
| Website: |
https://www.alfa-chemistry.com |
| Company Name: |
Musechem
|
| Tel: |
+1-800-259-7612 |
| Website: |
www.musechem.com |
|