| Identification | Back Directory | [Name]
abt199 int 2 | [CAS]
1235865-75-4 | [Synonyms]
abt199 int 2
ABT199 intermediates
ABT-199 Intermediate
ABT199 intermediate 3
Venetoclax Intermediate 3
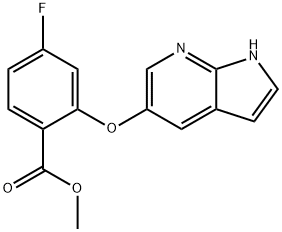
methyl 4-fluoro-2-{1H-pyrrolo[2,3-b]pyridin-5-yloxy}benzoate
Methyl 2-((1H-pyrrolo[2,3-b]pyrin-
5-yl)oxy)-4-fluorobenzoate
methyl 2-((1H-pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-fluorobenzoate
Methyl-2-((1H-pyrrolo[2,3-b]pyridine-5-yl)oxy)-4-fluorobenzoate
Benzoic acid, 4-fluoro-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)-, methyl ester | [Molecular Formula]
C15H11FN2O3 | [MDL Number]
MFCD28129691 | [MOL File]
1235865-75-4.mol | [Molecular Weight]
286.26 |
| Hazard Information | Back Directory | [Description]
Methyl 4-Fluoro-2-{1H-pyrrolo[2,3-b]pyridin-5-yloxy}benzoate(1235865-75-4) is a pharmaceutical intermediate compound that can be used in the preparation of a bcl-2 inhibitor intermediate, methyl 4-fluoro-2-((3-fluoro-lH-pyrrolo[2,3-b]pyridin-5-yl)oxy)benzoate. It can be used in the treatment of some cancers.
| [Chemical Properties]
Methyl 4-Fluoro-2-{1H-pyrrolo[2,3-b]pyridin-5-yloxy}benzoate appears as off-white solid, Store at 0-8 °C. | [Uses]
Methyl 4-Fluoro-2-{1H-pyrrolo[2,3-b]pyridin-5-yloxy}benzoate(1235865-75-4) is the intermediate for ABT 199 (A112430), which is a potent and selective BCL-2 inhibitor that achieves potent antitumor activity while sparing platelets. It’s practical application is to treat chronic lymphocytic leukaemic cells and estrogen receptor-positive breast cancer.
| [Synthesis]
Methyl 4-Fluoro-2-{1H-pyrrolo[2,3-b]pyridin-5-yloxy}benzoate(1235865-75-4) is prepared by the reaction of 5-Hydroxy-7-azaindole and Methyl 2,4-difluorobenzoate
. The specific synthesis steps are as follows:
To a three-necked flask was added 100 g of 5-hydroxy-7-azaindole (746 mmol), 141 g of methyl 2,4-difluorobenzoate(821 mmol), 237 g of potassium phosphate (1.12 mol) and 500 mL of diethylene glycol dimethyl Ether, 110 ° C for 24 h (HPLC to monitor 5-hydroxy-7-azaindole).The reaction solution was concentrated to dryness, and 2L of ethyl acetate and 2 L of water were added. The organic phase was separated,dried over anhydrous sodium sulfate, and concentrated to dry crude.The crude product was heated to reflux with 1260 mL of ethyl acetate. The mixture was slowly added dropwise to a solution of 1260 mL ofpetroleum ether. After 1 h of dropping, the mixture was stirred for 1 h, slowly cooled to 25 ° C, filtered and dried to give163g of pale white solidRate of 76.5percent.HPLC purity 98percent.
![Methyl 4-Fluoro-2-{1H-pyrrolo[2,3-b]pyridin-5-yloxy}benzoate synthesis Methyl 4-Fluoro-2-{1H-pyrrolo[2,3-b]pyridin-5-yloxy}benzoate synthesis](/NewsImg/2024-04-03/6384773502334631747821437.png)
|
|
|






![Methyl 4-Fluoro-2-{1H-pyrrolo[2,3-b]pyridin-5-yloxy}benzoate synthesis Methyl 4-Fluoro-2-{1H-pyrrolo[2,3-b]pyridin-5-yloxy}benzoate synthesis](/NewsImg/2024-04-03/6384773502334631747821437.png)
