| Identification | More | [Name]
DIMETHYL TEREPHTHALATE | [CAS]
120-61-6 | [Synonyms]
DIMETHYL 1,4-BENEDICARBOXYLATE
Dimethyl p-phthalate
DIMETHYL TEREPHTHALATE
DMT
METHYL-TERE-PHTHALATE
RARECHEM AL BF 0108
TEREPHATHALIC ACID DIMETHYL ESTER
TEREPHTHALIC ACID,BIS-METHYL ESTER
TEREPHTHALIC ACID DIMETHYL ESTER
1,4-Benzenedicarboxylicacid,dimethylester
1,4-Benzenedicarboxylicaciddimethylester
di-meterephthalate
Dimethyl 1,4-benzenedicarboxylate
Dimethyl ester of 1,4-benzenedicarboxylic acid
Dimethyl p-benzenedicarboxylate
dimethyl1,4-benzenedicarboxylate
dimethyl4-phthalate
Dimethylester kyseliny isoftalove
Dimethylester kyseliny tereftalove
dimethylesterkyselinyisoftalove(czech) | [EINECS(EC#)]
204-411-8 | [Molecular Formula]
C10H10O4 | [MDL Number]
MFCD00008440 | [Molecular Weight]
194.18 | [MOL File]
120-61-6.mol |
| Chemical Properties | Back Directory | [Appearance]
white chunks | [Melting point ]
140 °C | [Boiling point ]
288 °C
| [bulk density]
500kg/m3 | [density ]
1,29 g/cm3 | [vapor density ]
1.04 (vs air)
| [vapor pressure ]
1.15 mm Hg ( 93 °C)
| [refractive index ]
1.4752 | [Fp ]
154 °C
| [storage temp. ]
Store below +30°C. | [solubility ]
water: slightly soluble0.0493g/L at 20°C | [form ]
Flakes or Pellets | [pka]
0[at 20 ℃] | [color ]
White | [Stability:]
Stable. Incompatible with strong acids, strong bases, strong oxidizing agents. | [explosive limit]
0.8-11.8%(V) | [Water Solubility ]
It is slightly soluble in water but soluble in hot alcohol and ether. | [Merck ]
14,9162 | [BRN ]
1107185 | [LogP]
2.25 at 25℃ | [CAS DataBase Reference]
120-61-6(CAS DataBase Reference) | [EPA Substance Registry System]
Dimethyl terephthalate (120-61-6) |
| Safety Data | Back Directory | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
3256 | [WGK Germany ]
1
| [RTECS ]
WZ1225000
| [Autoignition Temperature]
520 °C DIN 51794 | [TSCA ]
Yes | [HS Code ]
29173700 | [Safety Profile]
Moderately toxic by
intraperitoneal route. Mdly toxic by
ingestion. An eye irritant. Mutation data
reported. When heated to decomposition it
emits acrid smoke and irritating fumes | [Hazardous Substances Data]
120-61-6(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 3200 mg/kg |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Methanol-->Sulfuric acid-->Copper(II) sulfate-->P-XYLENE-->Terephthalic acid-->mono-Methyl terephthalate-->Methyl 4-methylbenzoate-->1,4-Benzenedicarboxylic acid 1-ethyl 4-methyl ester-->2,5-FURANDICARBOXYLIC ACID DIETHYL ESTER | [Preparation Products]
Polyester resin paint-->polyester-based liquid crystalling ionmer containing sulfonate group-->mono-Methyl terephthalate-->Polyethylene Terephthalate-->Antistatic finishing agent-->1,4-Cyclohexanedimethanol-->3,8,15,20,27,32-Hexaoxatetracyclo[32.2.2.210,13.222,25]dotetraconta-1(3-->TEREPHTHALIC ACID 2-HYDROXYETHYL METHYL ESTER-->TEREPHTHALIC ACID BIS(2-HYDROXYETHYL) ESTER-->P-XYLENE-ALPHA,ALPHA,ALPHA,ALPHA',ALPHA',ALPHA'-D6-->TRANS-1,4-CYCLOHEXANEDIMETHANOL |
| Hazard Information | Back Directory | [Description]
Dimethyl terephthalate (DMT) (120-61-6) is an organic compound with the formula C6H4(CO2CH3)2. It is the diester formed from terephthalic acid and methanol. It is a white solid that melts to give a distillable colourless liquid.
| [Chemical Properties]
The empirical formula of dimethyl terephthalate (DMT) is
C10H10O4. Its structural formula is 1,4-(COOCH3)2C6H4. At
room temperature, exists as colorless crystals. DMT is soluble in ether and
chloroform, slightly soluble in ethanol, and fairly insoluble in
water (<1 g/L at 13℃). | [Uses]
Dimethyl terephthalate is used in the production of polyesters, including polyethylene terephthalate (PET) and poly trimethylene terephthalate. It consists of benzene substituted with carboxy methyl groups (CO2CH3) at the 1 and 4 positions. Because DMT is volatile, it is an intermediate in some schemes for the recyclic of PET, e.g. from plastic bottles.
Hydrogenation of DMT affords the diol cyclohexanedimethanol, which is a useful monomer. | [Uses]
Dimethyl terephthalate is used widely as an industrial intermediate to manufacture polyethylene terephthalate (PET) and dioctyl terephthalate (OECD, 2001).It is used in the Polyester Film (Audio/Video Tape,X-ray Film,Photo Film), Polyester Fiber, Pet Bottle, Polyester Adhesive, Engineering Plastics. DMT is volatile, it is an intermediate in some schemes for the recycling of PET. | [Application]
Dimethyl terephthalate undergoes enzymatic polycondensation with 1,8-diaminooctane to yield oligo(octamethylene terephthalamide).
Dimethyl terephthalate was used to synthesize silicone aromatic polyesters by the transesterification reaction with α,ω-bis(hydroxyalkyl)-terminated poly(dimethylsiloxane) in toluene. It was used in the synthesis of multiblock copolymers consisting of polyiso-butylene, dimethyl terephthalate and 1,4-butanediol. | [Definition]
ChEBI: Dimethyl terephthalate is a diester resulting from the formal condensation of the carboxy groups of terephthalic acid with methanol. It is a primary ingredient widely used in the manufacture of polyesters and industrial plastics. It is a methyl ester, a diester and a phthalate ester. It derives from a terephthalic acid. | [Preparation]
Several processes have been developed for the preparation of dimethyl
terephthalate from p-xylene, but the most important proceeds as follows:
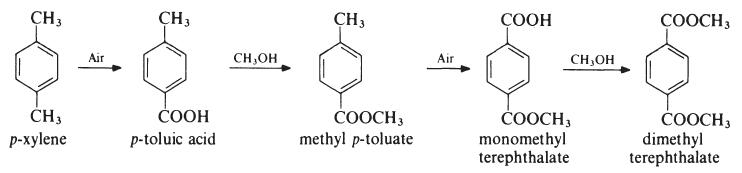
The oxidation steps are carried out in the liquid phase at about 170??C and
1.5 MPa (15 atmospheres) in the presence of a cobalt acetate or naphthenate
catalyst whilst the esterifications are conducted at about 150??C.
Dimethyl terephthalate may also be produced by esterification of terephthalic
acid. | [Production Methods]
Dimethyl terephthalate (DMT) has been produced in a number of ways. Conventionally and still of commercial value is the direct esterification of terephthalic acid. Alternatively, it can be prepared by alternating oxidation and methyl-esterification steps from p-xylene via methyl-p-toluate. | [Synthesis Reference(s)]
Chemistry Letters, 15, p. 851, 1986
Journal of the American Chemical Society, 111, p. 8742, 1989 DOI: 10.1021/ja00205a039
Tetrahedron Letters, 17, p. 3299, 1976 | [General Description]
Dimethyl terephthalate appears as white solid or heated colorless liquid. Has no odor. Liquid solidifies in cool water. Solid and liquid sink in water. (USCG, 1999) | [Air & Water Reactions]
When mixed with air, the vapor or dust forms very hazardous and highly reactive mixtures. . Insoluble in water. | [Reactivity Profile]
Dimethyl terephthalate(120-61-6) is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Can generate electrostatic charges. [Handling Chemicals Safely 1980. p. 250]. Dimethyl terephthalate(120-61-6) is sensitive to heat. The molten material reacts with water due to the temperature. Dimethyl terephthalate(120-61-6) is incompatible with strong oxidizers, strong acids and strong bases. | [Hazard]
H317 (56.25%): May cause an allergic skin reaction [Warning Sensitization, Skin]
H319 (14.06%): Causes serious eye irritation [Warning Serious eye damage/eye irritation]
H412 (29.69%): Harmful to aquatic life with long lasting effects [Hazardous to the aquatic environment, long-term hazard] | [Health Hazard]
Molten DMT will cause severe burns of skin on contact. | [Fire Hazard]
DIMETHYL TEREPHTHALATE is combustible. | [Potential Exposure]
Tumorigen,Mutagen. Primary Irritant. Essentially all DMT is consumedin the production of polyethylene terephthalate, the polymerfor polyester fibers and polyester films. Less than 2% ofproduction is used to make polybutylene terephthalateresins and other specialty products | [First aid]
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. | [Carcinogenicity]
In a study conducted by the
NCI, DMT was not considered to be carcinogenic in
rats or mice ingesting 2500 or 5000 ppm in the diet for
103 weeks. | [Source]
Dimethyl terephthalate is a natural product found in Hypotrachyna nepalensis, Uncaria elliptica, and other organisms with data available. | [storage]
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with DMT youshould be trained on its proper handling and storage. Storein tightly closed containers in a cool, well-ventilated areaaway from oxidizers, nitrates, acids,Wear protective gloves andclothing to prevent any reasonable probability of skin contact. Safety equipment suppliers/manufacturers can providerecommendations on the most protective glove/clothingmaterial for your operation. All protective clothing (suits,gloves, footwear, headgear) should be clean, available eachday, and put on before work. Contact lenses should not beworn when working with this chemical. Wear dust-proofchemical goggles and face shield unless full face-piecerespiratory protection is worn. Employees should washimmediately with soap when skin is wet or contaminated.Provide emergency showers and eyewash. and sources of ignition.A regulated, marked area should be established where thischemical is handled, used, or stored in compliance withOSHA Standard 1910.1045. | [Shipping]
Wear protective gloves andclothing to prevent any reasonable probability of skin contact. Safety equipment suppliers/manufacturers can providerecommendations on the most protective glove/clothingmaterial for your operation. All protective clothing (suits,gloves, footwear, headgear) should be clean, available eachday, and put on before work. Contact lenses should not beworn when working with this chemical. Wear dust-proofchemical goggles and face shield unless full face-piecerespiratory protection is worn. Employees should washimmediately with soap when skin is wet or contaminated.Provide emergency showers and eyewash. | [Purification Methods]
Purify it by recrystallisation from aqueous EtOH, MeOH or CCl4; or by zone melting. [Beilstein 6 H 843, 6 III 4250, 6 IV 3303.] . | [Incompatibilities]
Incompatible with strong acids, nitrates,strong oxidizers. |
|
|