| Identification | More | [Name]
2,6-NAPHTHALENEDICARBOXYLIC ACID | [CAS]
1141-38-4 | [Synonyms]
2,6-NAPHTHALENEDICARBOXYLIC ACID
2,6-NAPHTHALIC ACID
NAPHTHALENE-2,6-DICARBOXYLIC ACID
TIMTEC-BB SBB008377
2,6-NAPHTHALENEDICARBOXYLIC ACID, 99.5+%
2,6-NAPHTHALENEDICARBOXYLIC ACID 98+%
Naphthalene-2,6-dicarboxylic acid, 98+%
Naphthalene-2,6-dicarboxylic acid 99%
2,6-NAPTHALENEDICARBOXYLIC ACID
Naphthaline-2,6-dicarbonic acid | [EINECS(EC#)]
214-527-0 | [Molecular Formula]
C12H8O4 | [MDL Number]
MFCD00004105 | [Molecular Weight]
216.19 | [MOL File]
1141-38-4.mol |
| Chemical Properties | Back Directory | [Appearance]
BEIGE POWDER | [Melting point ]
>300 °C (lit.) | [Boiling point ]
316.6°C (rough estimate) | [density ]
1.5
| [refractive index ]
1.7080 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
very faint turbidity in hot Pyridine | [form ]
Powder | [pka]
3.69±0.30(Predicted) | [color ]
white | [Water Solubility ]
3μg/L at 20℃ | [BRN ]
2051257 | [InChI]
InChI=1S/C12H8O4/c13-11(14)9-3-1-7-5-10(12(15)16)4-2-8(7)6-9/h1-6H,(H,13,14)(H,15,16) | [InChIKey]
RXOHFPCZGPKIRD-UHFFFAOYSA-N | [SMILES]
C1=C2C(C=C(C(O)=O)C=C2)=CC=C1C(O)=O | [LogP]
2.22 | [CAS DataBase Reference]
1141-38-4(CAS DataBase Reference) | [EPA Substance Registry System]
2,6-Naphthalenedicarboxylic acid (1141-38-4) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [TSCA ]
T | [HazardClass ]
IRRITANT | [HS Code ]
29173990 |
| Hazard Information | Back Directory | [Chemical Properties]
BEIGE POWDER | [Uses]
2,6-naphthalenedicarboxylic acid is an important product for the manufacture of high strength and excellent dyeing properties of polyester fibers and F class insulation material, is an important monomer performance PEN, PBN, liquid crystal polymer (LCP) and polyurethane resin pharmaceutical and fine chemicals raw material. | [Uses]
A polybasic acid intended for use as components of resinous and polymeric coatings that contact food. | [Definition]
ChEBI: Naphthalene-2,6-dicarboxylic acid is a dicarboxylic acid. It derives from a hydride of a naphthalene. | [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here. | [Flammability and Explosibility]
Notclassified | [Synthesis]
2,6-Naphthalenedicarboxylic acid (2,6-NDA) is selectively synthesized from 2-naphthoic acid (2-NCA) and carbon tetrachloride in aqueous sodium hydroxide solution under mild conditions using cyclodextrin (β-CyD) as a catalyst[1]. The specific reaction steps are as follows:
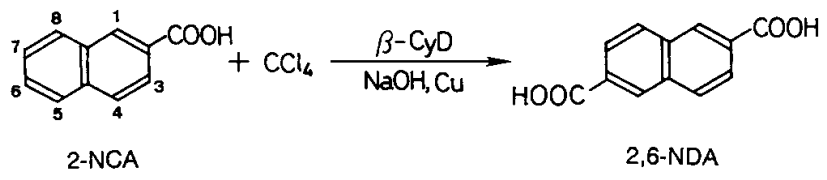
Three mmol of 2-naphthalenecarboxylic acid/2-NCA), 0,8 mmol(0,05 g) of copper powder, and 3,0 mmol of β-CyD were added to 30 mL of 30 wt.-% aqueous sodium hydroxide solution. The reaction was started with the addition of 8,5 mmol of carbon tetrachloride and was continued at 60°C for 7 h with magnetic stirring under nitrogen. Then, the residual carbon tetrachloride was removed by evaporation under reduced pressure.
| [References]
[1] HIDEFUMI HIRAI; Rikinori T; Hisashi Mihori. Selective synthesis of 2,6-naphthalenedicarboxylic acid using cyclodextrin as catalyst[J]. Macromolecular Rapid Communications, 1993, 14 7: 439-443. DOI:10.1002/marc.1993.030140712. |
|
|