| Identification | More | [Name]
4-Hydroxy-3-methoxycinnamic acid | [CAS]
1135-24-6 | [Synonyms]
(2E)-3-(4-HYDROXY-3-METHOXYPHENYL)ACRYLIC ACID
3-(4-HYDROXY-3-METHOXYPHENYL)ACRYLIC ACID
3-METHOXY-4-HYDROXYCINNAMIC ACID
4-HYDROXY-3-METHOXYCINNAMIC ACID
AKOS BBS-00006472
AURORA 17401
BUTTPARK 121\04-54
(E)-3-(4-HYDROXY-3-METHOXY-PHENYL)-ACRYLIC ACID
FERULIC ACID
FERULLIC ACID
LABOTEST-BB LT00453064
OTAVA-BB BB7016261120
RARECHEM BK HW 0087
TIMTEC-BB SBB000326
TRANS-4-HYDROXY-3-METHOXYCINNAMIC ACID
TRANS-FERULIC ACID
2-Propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)-
3-(4-Hydroxy-3-methoxyphenyl)-2-propenoic acid
3-(4-hydroxy-3-methoxyphenyl)-2-propenoicaci
3-(4-hydroxy-3-methoxyphenyl)-2-Propenoicacid | [EINECS(EC#)]
208-679-7 | [Molecular Formula]
C10H10O4 | [MDL Number]
MFCD00004400 | [Molecular Weight]
194.18 | [MOL File]
1135-24-6.mol |
| Chemical Properties | Back Directory | [Definition]
A plant growth
inhibitor. | [Appearance]
Pale Yellow Solid | [Melting point ]
168-172 °C(lit.)
| [Boiling point ]
250.62°C (rough estimate) | [density ]
1.316(20.0000℃) | [vapor pressure ]
0Pa at 25℃ | [refractive index ]
1.5168 (estimate) | [storage temp. ]
2-8°C | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
powder
| [pka]
4.58±0.10(Predicted) | [color ]
slightly yellow
| [Water Solubility ]
soluble | [Usage]
Widely distributed in small amounts in plants. Used as an antioxidant and food preservative | [InChIKey]
KSEBMYQBYZTDHS-HWKANZROSA-N | [LogP]
1.51 | [Uses]
ferulic acid is a plant-derived anti-oxidant and free-radical scavenger, it protects the skin against uVB-induced redness. When incorporated into formulas with ascorbic acid and tocopherol, ferulic acid can improve their stability and double the photoprotection capacities offered by the formulation. In clinical studies, ferulic acid exhibits good permeation capacities through the stratum corneum, which can be attributed to its lipophilic properties. | [CAS DataBase Reference]
1135-24-6(CAS DataBase Reference) | [NIST Chemistry Reference]
Cinnamic acid, 4-hydroxy-3-methoxy-(1135-24-6) | [EPA Substance Registry System]
1135-24-6(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
3
| [RTECS ]
UD3365500
| [Hazard Note ]
Irritant | [HazardClass ]
IRRITANT | [HS Code ]
29189900 | [Hazardous Substances Data]
1135-24-6(Hazardous Substances Data) | [Toxicity]
mouse,LD,intraperitoneal,> 350mg/kg (350mg/kg),BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY)BEHAVIORAL: ATAXIABEHAVIORAL: REGIDITY,Indian Journal of Pharmaceutical Sciences. Vol. 49, Pg. 77, 1987. |
| Hazard Information | Back Directory | [Description]
Ferulic acid is widely found in plants, especially in artichoke, eggplant and corn
bran. In addition, it is also present in a variety of Chinese herbal medicines, such as
angelica, dome, motherwort, snow ganoderma lucidum and so on. | [Chemical Properties]
Ferulic acid is a pale yellow solid, It belongs to the family of hydroxycinnamic acids. It is an abundant phenolic phytochemical found in plant cell wall components. Natural sources of ferulic acid are leaves and seeds of many plants, such as cereals, coffee, apples, artichokes, peanuts, oranges, pineapples and wine. | [Physical properties]
Appearance: light yellow crystalline powder. Solubility: slightly soluble in cold
water; soluble in hot water, with poor stability in aqueous solution; easily decomposed when encounter light; soluble in ethanol and ethyl acetate; slightly soluble in
ether; insoluble in benzene and petroleum ether. Melting point: 170–173?°C. | [Indications]
This product is mainly used for the treatment of atherosclerosis, coronary heart
disease and ischemic cerebrovascular disease. | [Biotechnological Production]
There are three different natural sources for ferulic acid. It could be produced from
low-molecular-weight ferulic conjugates. For example, ferulic acid has been
isolated from the waste material of rice bran oil production by hydrolyzing with
sodium hydroxide or potassium hydroxide at 90–100 �C. Ferulic acid with a purity
of 70–90 % was produced within 8 h under atmospheric pressure
Another possibility is a direct extraction of ferulic acid from plant cell walls by
using feruloyl esterases. Various microorganism are able to secrete feruloyl
esterases (e.g. A. niger, Bacillus species and Clostridium thermocellum). The
enzymatic hydrolysis of sugar-beet pulp has been analyzed using a mixture of
carbohydrases from Aspergillus aculeatus with a final ferulic acid concentration of
200 mg.L-1 in the hydrolyzate. Moreover, a purification method to isolate
ferulic acid from sugar-beet pulp after enzymatic hydrolysis using a fixed-bed
adsorption with activated carbon has been developed. With this process, a purity of
50 % has been achieved. Finally, ferulic acid could be produced by cell
culture fermentations. For example, free ferulic acid (up to 50 mg.L-1) and
also conjugated to anthocyanins (up to 150 mg.L-1) has been accumulated in cell
cultures of Ajuga pyramidalis. | [Pharmacokinetics]
In clinical practice, Honghua and clopidogrel are often combined with FA-containing herbs to treat cardiovascular disease. Li et al. found that Ferulic acid (FA) was rapidly absorbed with a low bioavailability after a single oral administration. The pharmacokinetics profile of FA in rats was partly altered by the coadministration of FA with Honghua or clopidogrel. FA was rapidly absorbed following oral administration with a mean time to peak plasma concentration (T(max)) of 0.03 h. The corresponding maximum plasma concentration (C(max)) and the area under the concentration-time curve (AUC) were 8174.55 ng/L and 2594.45 h ng/mL, respectively. Coadministration of Honghua and clopidogrel resulted in a 63.5% and 79.7% increase in the AUC, respectively. The C(max) of FA was significantly increased by coadministration with clopidogrel (74.3%, p<0.01). Moreover, the T(max) of FA when coadministered with Honghua or clopidogrel was 3 and 3.76 times slower than when administered alone. The coadministrations also altered other pharmacokinetic parameters estimated for FA, but no statistically significant differences were observed[1]. | [Pharmacology]
Orally administered ferulic acid completely prevents the formation of skin tumors, reverts the status of phase I and phase II detoxication agents, lipid peroxidaton byproducts and antioxidants to near-normal ranges in 7,12-DMBA-treated mice (Alias et al., 2009). The observation demonstrate that orally administered ferulic acid has potent suppressive effects on cell proliferation during DMBA-induced skin carcinogenesis.
Ferulic acid also has the capacity to prevent UV-induced damage to cells. Ferulic acid is often added as an ingredient to anti-aging supplements. When ferulic acid was incorporated into a formulation of α-tocopherol and/or ascorbic acid, the topical delivery of the vitamins was improved. There was enhanced chemical stability and the photoprotection to solar-simulated irradiation doubled (Lin et al., 2005; Cassano et al., 2009). For example, Murray et al. (2008) applied a stable topical formulation (containing 1% α-tocopherol, 15% L-ascorbic acid, and 0.5% ferulic acid) to normalappearing human skin and a pig skin model. These were then irradiated with solar-simulated UV. The results showed the complex of antioxidants provided substantial UV photoprotection against erythema, sunburnt cells, thymine dimmers, p53 as well as UV-induced cytokine formation including IL-1α, IL-6, IL-8, and IL-10, and TNF-α (Murray et al., 2008). | [Clinical Use]
At present, there are sodium ferulate tablets and ferulic acid injection used in clinic.
Sodium ferulate tablets are mainly used for the adjuvant therapy of atherosclerosis,
coronary heart disease, cerebrovascular disease, glomerular disease, pulmonary
hypertension, diabetic vascular disease, vasculitis and other vascular disorders.
Ferulic acid can also be used for the treatment of migraine headache and vascular
headache. Ferulic acid injection is mainly used for the treatment of ischemic cardiovascular and cerebrovascular disease. In addition, sodium ferulate combined with
atorvastatin can be used for the treatment of pulmonary hypertension, diabetic
nephropathy and chronic glomerulonephritis in clinic .
Ferulic acid is also used in combination with other drugs to treat other diseases. | [Side effects]
Ferulic acid serums and creams are generally safe for most skin types. However, it's not safe for everyone.
Sensitive skin. This can cause: Mild redness, Irritation.
Allergic to bran or oatmeal. People may also have an allergy to ferulic acid serums derived from bran or oats. Symptoms tend to be mild and may include: Redness, Swelling, Itching, Rash, and Peeling. | [storage]
Store at 2-8°C | [Purification Methods]
Crystallise ferulic acid from H2O. [Beilstein 10 H 436, 10 IV 1776.] | [References]
[1] Wang, Mi Ningsheng . "Pharmacokinetics of ferulic acid and potential interactions with Honghua and clopidogrel in rats." Journal of Ethnopharmacology (2011). |
| Questions And Answer | Back Directory | [Plant sources]
Ferulic acid is a kind of phenolic acid extracted from the resin of ferula asafetida. Ferula asafetida is a kind of Umbelliferae perennial herb with a strong garlic smell and living in sandy areas. It is mainly produced in Xinjiang. During the nascent stage, there are only root leaves. At 5 years, scape emerges. The scape is very strong with its height being up to two meters. At end spring and early summer (flowering stage to early fruit stage), apply slanting cut from the upper position down to the bottom separately, collect oozing milky resin, dry it. Ferula asafetida contains volatile oils, gums and resins with oil containing various kinds of organic acids such as (R)-sec-butyl-1-propenyl disulfide, 1(1-methylthio-propyl) 1-propenyl disulfide, sec-butyl 3-methylthio-allyl-disulfide. Resin containing ferulic acid and its related esters.
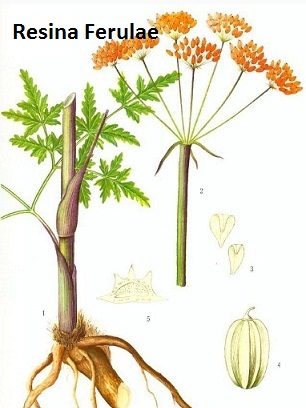
Figure 1 is Resina Ferulae
| [Physical and Chemical Properties]
Ferulic acid is an aromatic acid widely being presented in plant kingdom and is the components of suberin. It amount is very small presented in plants in its free state but with its main form in forming bound state with oligosaccharides, polyamines, lipids and polysaccharides. It has many health functions, such as free radical scavenging, anti-thrombotic, anti-inflammatory, anti-tumor, prevention and treatment of hypertension, heart disease, and enhanced sperm activity and so on. Ferulic acid has a low toxicity and is easy for being metabolized by human. It can be used as a food preservative and has a wide range of applications in the field of food and medication.
The above information is edited by the chemicalbook of Yan Yanyong.
| [History]
Ferulic acid is a derivative of cinnamic acid with molecular formula C10H10O4. In 1886, Hlasiwetz Barth, an Austrian, isolated 3-methoxy-4-hydroxycinnamic acid from the genus Ferula foetida for structure determination. Ferulic acid together with dihydroferulic acid, is a component of lignocelluloses, conferring cell wall rigidity by cross linking lignin and polysaccharides. It is commonly found in seeds of plant such as rice, wheat and oats. Besides, Ferulic Acid exhibited biochemical role in the inhibition of seed germination, inhibition of indole-acetic acid and enzyme, inhibition of decarboxylation activity & other protective effect on micro-organisms and pets.
The syntheis of Ferulic acid was established by Dutt in 1935 when ferulic acid was used as a precursor in the manufacturing of vanillin and malonic acid. There are vast numbers of studies documented on the bio-medical properties of ferulic acid such as antioxidant activity, UV-absorbing capacity & its effect of lignin as precursor in plants metabolic pathway. Ferulic acid, being highly abundant, is indeed difficult to synthesize, Oryza Oil & Fat Chemical has successfully developed an efficient method to extract ferulic acid from rice bran and suitable for applications in the health and beauty arena.
| [Lipid-Lowering effect]
Ferulic acid can competitively inhibit the liver mevalonate-5-pyrophosphate dehydrogenase activity, inhibiting the synthesis of cholesterol in the liver, so as to achieve the purpose of lowering blood pressure.
| [Antimicrobial effect]
Ferulic acid exhibits a broader anti-bacterial spectrum. It has been found that ferulic acid is able to inhibit pathogenic bacteria such as Shigella sonnei, Klebsiella pneumoniae, Enterobacter, Escherichia coli, Citrobacter, Pseudomonas aeruginosa and 11 kinds of microorganisms which causing food corruption.
| [Food Industry Applications]
In addition to its wide application in medicine, ferulic acid has been approved by some countries to be as a food additive. Japan has approved it to be used in food antioxidants while the United States and some European countries have allowed for adopting some kinds of herbs, coffee, beans with relative high amount of ferulic acid for being antioxidant. Ferulic acid, in the food industry, is mainly used for the preparation of natural vanillin, antioxidants, preservatives, cross-linkers and functional promoting agent. The information is edited by Xiaonan from Chemicalbook.
| [Pharmacological effects]
Ferulic acid has various effects of inhibiting platelet aggregation, expectorant, and inhibition of Mycobacterium tuberculosis and so on. Clinically ferulic acid is mainly applied to the adjuvant treatment of various kinds of vascular diseases such as atherosclerosis, coronary heart disease, cerebrovascular, renal disease, pulmonary hypertension, diabetic vascular disease, and vasculitis as well as neutropenia and thrombocytopenia. It can be used for treating migraine and vascular headache. As a leukocyte-enhancement drug, this drug also has enhanced hematopoietic function. Therefore, ferulic acid may also be for the treatment of leukopenia and thrombocytopenia.
Reference: Xu Jingfeng, Yang Ming (editor) Handbook of clinical prescription drugs. Nanjing: Jiangsu Science and Technology Press .2009 on page 561.
| [Synthetic method]
Ferulic acid can be obtained through chemical synthesis and extraction. Laboratory dissolves the vanillin, malonic acid and piperidine in pyridine for reaction of three weeks after which with hydrochloric acid precipitation, you can obtain ferulic acid.
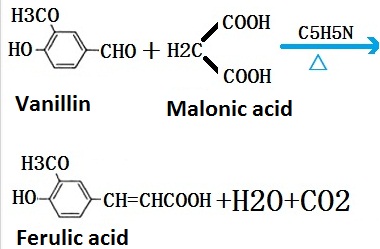
Figure 2 laboratory synthesis roadmap of ferulic acid
| [Uses]
It can be used as a food preservative and a kind of organic chemicals.
It can be used as the intermediates of cinametic acid. It can also be used as food preservative.
It can also be applied to biochemical studies
|
|
|