| Identification | More | [Name]
Calcipotriene | [CAS]
112965-21-6 | [Synonyms]
24-CYCLOPROPYL-(1ALPHA,3BETA,5Z,7E,22E,24S)-9,10-SECOCHOLA-5,7,10(19),22-TETRAENE-1,3,24-TRIOL
CALCIPOTRIENE
CALCIPOTRIOL
5z,7e,22e,24s)--bet
9,10-secochola-5,7,10(19),22-tetraene-1,3,24-triol,24-cyclopropyl-,(1-alpha,3
dovonex
mc903
CALCIPOTRIOL,CALCIPOTRIENE
Calciptriol, MC-903, Daivonex, Dovonex, Psorcutan
9,10-Secochola-5,7,10(19),22-tetraene-1,3,24-triol, 24-cyclopropyl-, (1α,3β,5Z,7E,22E,24S)-
Calciptriol
Daivobet
Daivonex
calcipotriol anhydre ep5.3
(1S,3S,5Z)-5-[(2E)-2-[(1R,3aR,7aR)-1-[(E,2S)-5-Cyclopropyl-5-hydroxy-pent-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidene-cyclohexane-1,3-diol
Calcipotriene
CALCIPOTRIOL-EP
(1�,3��,5Z�����,7E,22E�,24S)-24-Cyclopropyl-9,10-secochola-5�,7,10(19)���,22-tetraene-1�,3�����,24-triol
(1R,3S)-5-[2-[(1R,3aR,7aS)-1-[(2S)-5-cyclopropyl-5-hydroxy-pent-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidene-cyclohexane-1,3-diol
9,10-Secochola-5,7,10(19),22-tetraene-1,3,24-triol, 24-cyclopropyl-, (1-alpha,3-beta,5Z,7E,22E,24S) | [EINECS(EC#)]
601-218-4 | [Molecular Formula]
C27H40O3 | [MDL Number]
MFCD00866630 | [Molecular Weight]
412.6 | [MOL File]
112965-21-6.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
166-168°C | [Boiling point ]
582.0±50.0 °C(Predicted) | [density ]
1.12±0.1 g/cm3(Predicted) | [storage temp. ]
Desiccate at -20°C | [solubility ]
DMSO: soluble15mg/mL, clear | [form ]
powder | [pka]
14.29±0.20(Predicted) | [color ]
White to off-white | [Usage]
An antipsoriatic. A vitamin D3 analogue with low calcemic activity | [Stability:]
Light and Temperature Sensitive, Light And Temperature Sensitive | [InChIKey]
LWQQLNNNIPYSNX-HCHVWAPNSA-N | [SMILES]
[C@@H]1(O)C/C(=C/C=C2\CCC[C@@]3(C)[C@@]\2([H])CC[C@@H]3[C@H](C)/C=C/[C@H](C2CC2)O)/C(=C)[C@@H](O)C1 | [CAS DataBase Reference]
112965-21-6(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
36/37/38 | [Safety Statements ]
26 | [RIDADR ]
UN 2811 6.1 / PGIII | [WGK Germany ]
3 | [HS Code ]
2906195000 | [Toxicity]
dog,LD50,skin,> 1500ug/kg (1.5mg/kg),BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)MUSCULOSKELETAL: OTHER CHANGESGASTROINTESTINAL: NAUSEA OR VOMITING,Journal of Toxicological Sciences. Vol. 21(Suppl, |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Selenium dioxide-->tert-Butyldimethylsilyl chloride-->4-METHYLMORPHOLINE-4-OXIDE SOLUTION-->Tetrabutylammonium fluoride-->Anthracene-->Cyclopropylmagnesium bromide-->Triethylamine-->Sodium borohydride-->CERIUM(III) CHLORIDE-->PHOSPHINE OXIDE, [2-[3,5-BIS [[(1,1-DIMETHYLETHYL) DIMETHYLSILY]OXY]-2-METHYLENECYCLOHEXYLIDENE]ETHYL]DIPHENYL-,[3S-(1Z,3A,5B0)]-->(1S,4R,E)-4-((1R,3aS,7aR,E)-4-((Z)-2-((3S,5R)-3,5-bis((tert-butyldiMethylsilyl)oxy)-2-Methylenecyclohexylidene)ethylidene)-7a-Methyloctahydro-1H-inden-1-yl)-1-cyclopropylpent-2-en-1-ol-->(5E)-Calcipotriene | [Preparation Products]
24R-Calcipotriol |
| Hazard Information | Back Directory | [Description]
Calcipotriol is a topical vitaminD3 derivative effective in the treatment of psoriasis
vulgaris. The drug acts by binding to vitamin D3 receptors in the skin keratinocytes,
producing an elevation in cell differentiation and a reduction in cell proliferation.
Although its efficacy is comparable to calcitriol, calcipotriol exhibits at least 100 times
less effect on calcium metabolism in rats. | [Chemical Properties]
White Crystalline Solid | [Originator]
Leo Denmark (Denmark) | [Uses]
A labelled antipsoriatic. A vitamin D3 analogue with low calcemic activity. | [Uses]
An antipsoriatic. A vitamin D3 analogue with low calcemic activity | [Uses]
antipsoriatic;viamin D receptor (VDR) affinity | [Definition]
ChEBI: Calcipotriol is a seco-cholestane that is 26,27-cyclo-9,10-secocholesta-5,7,10,22-tetraene carrying additional hydroxy substituents at positions 1, 3 and 24. It is used (as its hydrate) in combination with betamethasone dipropionate, a corticosteroid, for the topical treatment of plaque psoriasis in adult patients. It has a role as a drug allergen and an antipsoriatic. It is a member of cyclopropanes, a secondary alcohol, a triol, a hydroxy seco-steroid and a seco-cholestane. | [Indications]
Calcipotriene (Dovonex), a synthetic vitamin D3 derivative,
is indicated for the treatment of moderate plaque
psoriasis. Its mechanism of action is unknown, although
it competes for calcitriol receptors on keratinocytes and
normalizes differentiation. It also has a variety of immunomodulatory
effects in the skin. Although the drug
can cause local irritation, the most serious toxicities are
hypercalciuria and hypercalcemia, which are usually reversible. | [Preparation]
A convergent approach for the total synthesis of calcipotriol (brand name: Dovonex), a proven Vitamin D analog used for the treatment of psoriasis, and medicinally relevant synthetic analogs is described.
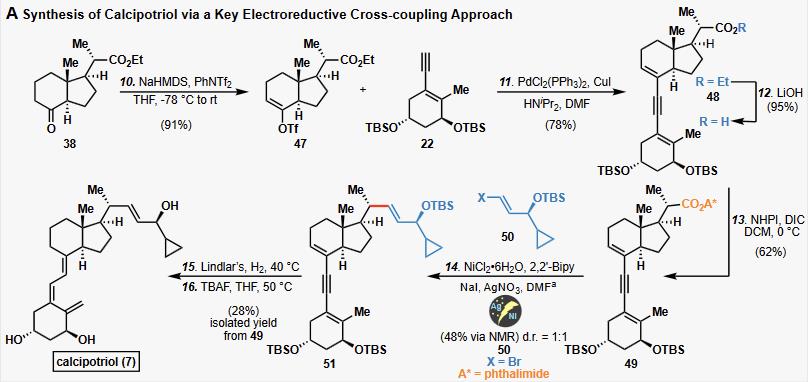
A Synthesis of calcipotriol via a Key Electroreductive Cross-coupling Approach
Calcipotriol is currently the most successful VitD analog and is prescribed for the treatment of psoriasis, an autoimmune skin disease. | [Manufacturing Process]
(1S,3R)-Bis-(t-butyldimethylsilyloxy)-(20S)-formyl-9,10-secopregna(5E,7E,10
(19))triene (Calverley Tetrahedron 43.4609 (1967) and (cyclopropyl)(tri-phenylphoshoranylidene)ketone are stirred in dimethyl sulfoxide under
nitrogen. The reaction mixture is then diluted at room temperature with ethyl
acetate and washed with common salt solution. The organic phase is dried on
sodium sulfate and filtered. After removal of the solvent, the residue is filtered
with toluene through silica gel. Evaporation of the solvent and gradient
chromatography (toluene/hexane (1:1)-toluene) of the residue on silica gel
yield (5E,7E,22E),(1S,3R)-1,3-bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-
9,10-secochola-5,7,10(19),22-tetraene-24-one.
(5E,7E,22E),(1S,3R)-1,3-Bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-9,10-
secochola-5,7,10(19),22-tetraene-24-one in tetrahydrofuran and methanol are
mixed with a 0.4 M methanol CeCl3·7H2O solution. Sodium borohydride is
added by portions under nitrogen with ice cooling. The suspension is stirred
with ice cooling and then put into ice/common salt solution. The aqueous
phase is extracted with ethyl acetate, the organic phase is washed neutral
with water and dried on sodium sulfate. Filtration and removal of the solvent
yield oil. By chromatography on silica gel with ethyl acetate/hexane (1:9). The
(5E,7E,22E),(1S,3R,24S)-1,3-bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-
9,10-secochola-5,7,10(19),22-tetraene-24-ol is obtained.
(5E,7E,22E),(1S,3R,24S)-1,3-Bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-
9,10-secochola-5,7,10(19),22-tetraene-24-ol is dissolved in toluene and after
addition of anthracene and 1 drop of triethylamine it is radiated at room
temperature with a high pressure mercury vapor lamp (Heraeus TQ 150)
through Pyrex glass. The reaction mixture is concentrated by evaporation and
the residue a mixture of (5Z,7E,22E),(1S,3R,24S)-1,3-bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-9,10-secochola-5,7,10(19),22-tetraene-
24-ol and anthracene - is directly reacted with tetrabutylammonium fluoride.
(5Z,7E,22E),(1S,3R,24S)-1,3-Bis-(t-butyldimethylsilyloxy)-24-cyclopropyl-
9,10-secochola-5,7,10(19),22-tetraene-24-ol in tetrahydrofuran is kept with a
1 M solution of tetrabutylammonium fluoride in tetrahydrofuran under
nitrogen. For working up, the cooled reaction mixture is poured into cold
sodium bicarbonate solution and then extracted with ethyl acetate. After
drying of the organic phase on sodium sulfate, filtration and evaporation of
the solvent yields a resin-like residue. Chromatography on silica gel with ethyl
acetate/hexane (2:1) yields (5Z,7E,22E),(1S,3R,24S)-24-cyclopropyl-9,10-
secochola-5,7,10(19),22-tetraene-1,3,24-triol. | [Brand name]
Dovonex (Leo);Daivonex. | [Therapeutic Function]
Antipsoriatic | [Biological Activity]
Vitamin D 3 analog that displays minimal effects on calcium homeostasis. Regulates cell differentiation and proliferation; exhibits antiproliferative activity against human HL-60, HL60/MX2, MCF-7, T47D, SCC-25 and mouse WEHI-3 cancer cell lines. | [Biochem/physiol Actions]
Calcipotriol, a synthetic derivative of calcitriol or Vitamin D, is used in the treatment of psoriasis and marketed under the trade name Dovonex. It has comparable affinity with calcitriol (Vit. D) for the Vitamin D receptor (VDR), while being less than 1% as active as the calcitriol in regulating calcium metabolism. VDR belongs to the steroid/thyroid receptor superfamily, and is found on the cells of many different tissues including the thyroid, bone, kindney, and T cells of the immune system. Binding of calcipotriol to the VDR modulates the T cells gene transcription of cell differentiation and proliferation-related genes. | [Pharmacology]
Calcipotriene (Dovonex) enhances the effectiveness of ultraviolet B
(UVB) but also increases photosensitivity in UVB-treated patients. Ultraviolet
light, 6% salicylic acid, 12% lactic acid, hydrocortisone valerate 0.2% ointment,
and tazarotene (Tazorac) gel degrade calcipotriene (Dovonex). Halobetasol
ointment and 5% tar gel are compatible with Calcipotriene (Dovonex).
A British study found calcipotriene (Dovonex) to be safe and effective in a
pediatric population over the age of 3, although it is not approved by the FDA. | [Side effects]
Possible side effects include:
severe burning, stinging, skin rash, or other irritation after applying the medicine;
worsening of your skin condition;
high calcium levels--confusion, tiredness, nausea, vomiting, loss of appetite, constipation, increased thirst or urination, weight loss;
mild skin irritation;
skin rash;
itching. | [storage]
Store at -20°C |
|
|