| Identification | More | [Name]
1,2-Decanediol | [CAS]
1119-86-4 | [Synonyms]
1,2-DECANEDIOL
1,2-DIHYDROXYDECANE
decane-1,2-diol
Decan-1,2-diol
1,2-Decanediol, GC 98%
1,2-Decandiol, 98%
1,2-DECANEDIOL 99+% | [EINECS(EC#)]
214-288-2 | [Molecular Formula]
C10H22O2 | [MDL Number]
MFCD00010739 | [Molecular Weight]
174.28 | [MOL File]
1119-86-4.mol |
| Chemical Properties | Back Directory | [Appearance]
White powder | [Melting point ]
48-50 °C (lit.) | [Boiling point ]
255 °C (lit.) | [density ]
0.9418 (rough estimate) | [vapor pressure ]
0.004Pa at 20℃ | [refractive index ]
1.4561 (estimate) | [Fp ]
113 °C | [storage temp. ]
-20°C Freezer | [solubility ]
Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
14.48±0.20(Predicted) | [color ]
White to Off-White | [Water Solubility ]
579mg/L at 20℃ | [InChI]
InChI=1S/C10H22O2/c1-2-3-4-5-6-7-8-10(12)9-11/h10-12H,2-9H2,1H3 | [InChIKey]
YSRSBDQINUMTIF-UHFFFAOYSA-N | [SMILES]
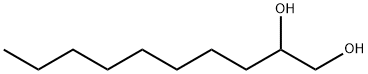
C(O)C(O)CCCCCCCC | [LogP]
1.99 at 22.4℃ | [CAS DataBase Reference]
1119-86-4(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
White powder | [Uses]
1,2-Decanediol is a 1,2-alkanediol that is used as an antimicrobial agent in cosmetics. 1,2-Decanediol also suppresses the fluidity of the hydrophilic and hydrophobic groups in the phospholipid membrane of liposomes. | [Definition]
ChEBI: 1,2-Decanediol is a glycol that is decane bearing two hydroxy substituents located at positions 1 and 2. It has a role as an anti-inflammatory agent, an antioxidant and a human metabolite. It is a glycol and a volatile organic compound. It derives from a hydride of a decane. | [Synthesis Reference(s)]
Journal of the American Chemical Society, 98, p. 1986, 1976 DOI: 10.1021/ja00423a067
Synthesis, p. 45, 1989 | [Flammability and Explosibility]
Nonflammable | [Synthesis]
Synthesis of 1,2-decanediol: A homogeneous clear solution is prepared by adding 3 moles of acetone to 1 mole of a mixture of 1-nonene oxide and 1-decene oxide at room temperature. After 50 grams of 5% aqueous sulfuric acid to the solution, the acetone is evaporated off and the resulting solution is neutralized by adding an aqueous sodium hydroxide. The neutralized solution is extracted with pet ether and the extract dried over anhydrous magnesium sulfate. The pet ether is stripped off and a mixture of 1,2-nonanediol and 1,2-decanediol is obtained by distilling under vacuo. |
|
|