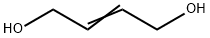| Identification | More | [Name]
2-Butene-1,4-diol | [CAS]
110-64-5 | [Synonyms]
1,4-BUTENEDIOL-[Z]
2-BUTENE-1,4-DIOL
butene-1,4-diol
CIS-1,4-DIHYDROXY-2-BUTENE
CIS-2-BUTENE-1,4-DIOL
(2E)-2-Butene-1,4-diol
1,4-dihydroxy-2-buten
2-Buten-1,4-diol
2-Butene, 1,4-dihydroxy-
2-Butene-1,4-diol,c&t
agrisynthb2d
Butenediol
2-BUTENE-1,4-DIOL (CIS-AND TRANS-MIXTURE)
but-2-ene-1,4-diol
1,4-buenedilo(liquid)
2-BUTENE-1,4-DIOL, 95% (PREDOMINANTLY CI
2-Butene-1,4-Diol,~92%
2-BUTENE-1,4-DIOL (CIS+TRANS)
1,4-Butendiol
2-Butene-1,4-diol | [EINECS(EC#)]
228-085-1 | [Molecular Formula]
C4H8O2 | [MDL Number]
MFCD00002924 | [Molecular Weight]
88.11 | [MOL File]
110-64-5.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [WGK Germany ]
3
| [RTECS ]
EM4970000
| [F ]
23 | [HS Code ]
29053990 | [Hazardous Substances Data]
110-64-5(Hazardous Substances Data) |
| Hazard Information | Back Directory | [Description]
2-Buten-1,4-diol is a palladium-containing compound that forms cross-links by reacting with an oxygen nucleophile. It reacts with nitrogen nucleophiles in the presence of hydrochloric acid to provide gamma-aminobutyric acid. This reaction mechanism is analogous to the way in which amines react with carboxylic acids to form amides. cis,trans-2-Buten-1,4-diol can be found in kinetic studies and its nmr spectra have been studied extensively. | [Chemical Properties]
Colorless to light yellow liquid | [Uses]
It is used to make agricultural chemicalsand the pesticide endosulfan; and as anintermediate for making vitamin B. | [Application]
2-Butene-1,4-diol has been used as a cross-linking agent for various polymers and is also able to form hydroxyl groups through allylation reactions. | [Reactivity Profile]
2-Butene-1,4-diol(110-64-5) forms furan (narcotic)when treated with dichromate in acidic solution.Dehydration of the cis-isomer overacid catalysts yields 2,5-dihydrofuran (narcotic).Halogens form substitution or additionproducts, 4-halobutenols, or 2,3-dihalo-1,4-butanediol. These are toxic compounds.Ammonia or amine form pyrroline or itsderivatives (moderately toxic). | [Health Hazard]
2-Butene-1,4-diol is a depressant of the Centralnervous system. Inhalation toxicity isvery low due to its low vapor pressure. Theoral LD50 value in rats and guinea pigs is1.25 mL/kg. It is a primary skin irritant. | [Fire Hazard]
Noncombustible liquid; flash point (open
cup) 128°C. |
|
|