| Identification | Back Directory | [Name]
3-(1-Naphthoyl)indole | [CAS]
109555-87-5 | [Synonyms]
-Naphthoyl Indole
1'-Naphthoyl Indole
3-(1-Naphthoyl)indole
Ketone indol-3-yl 1-naphthyl
Indol-3-yl 1-Naphthyl Ketone
3-(1-naphthylcarbonyl)indole
3- (1-Naphthoyl) Indole Powder
1H-Indol-3-yl-1-naphthalenylmethanone
Methanone, 1H-indol-3-yl-1-naphthalenyl-
(1H-Indol-3-yl)-naphthalen-1-yl-methanone
(1H-indol-3-yl)(naphthalene-1-yl)Methanone | [EINECS(EC#)]
207-791-3 | [Molecular Formula]
C19H13NO | [MDL Number]
MFCD12923093 | [MOL File]
109555-87-5.mol | [Molecular Weight]
271.318 |
| Chemical Properties | Back Directory | [Melting point ]
236℃ | [Boiling point ]
512.2±23.0 °C(Predicted) | [density ]
1.267 | [storage temp. ]
Refrigerator | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Solid | [pka]
15.22±0.30(Predicted) | [color ]
Pale Orange to Light Pink | [InChI]
InChI=1S/C19H13NO/c21-19(17-12-20-18-11-4-3-9-15(17)18)16-10-5-7-13-6-1-2-8-14(13)16/h1-12,20H | [InChIKey]
QIXQGVQLBPQVOR-UHFFFAOYSA-N | [SMILES]
C(C1C2=C(NC=1)C=CC=C2)(C1=C2C(C=CC=C2)=CC=C1)=O |
| Hazard Information | Back Directory | [Description]
3-(1-Naphthoyl)indole is a synthetic cannabinoid that is structurally similar to THC. It has been detected in human urine, plasma and autopsy samples by high-resolution mass spectrometry. It has been found to be hydroxylated into a number of metabolites including carboxy-3-(1-naphthoyl)indole, which may account for some of the psychoactive effects. | [Chemical Properties]
Pale Pink Solid | [Uses]
Metabolite of JWH-018 (P283650). | [Synthesis]
To a cooled solution of indole (5.85g, 50mmol) in ether (50ml) under nitrogen was added slowly a solution of methylmagnesium bromide (3M) in ether (17.5ml). After addition, the reaction mixture was warmed up to room temperature and stirred for 2h at room temperature. Then, the mixture was cooled down again to 0°C and added slowly, stirring a solution of 1-naphthoylchloride (9.5g, 50mmol) in ether (50ml). The resulting mixture was warmed up to room temperature and stirred for 2h at room temperature, followed by a slow addition of saturated ammonium chloride solution (375ml). The mixture was then stirred overnight at room temperature. A white solid was formed, filtered, washed by ether, and dried under a high vacuum to give 3-(1-Naphthoyl)indole (12.3g, 91%).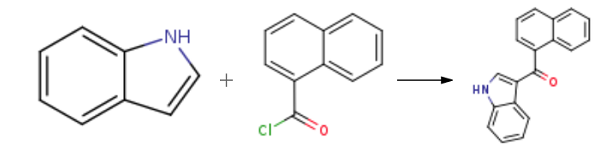
|
|
|