| Identification | More | [Name]
Phloroglucinol | [CAS]
108-73-6 | [Synonyms]
1,2,5-TRIHYDROXYBENZENE
1,3,5-BENZENETRIOL
1,3,5-THB
1,3,5-TRIHYDROXYBENZENE
1,3,5-TRIHYDROXYBENZENE ANH
3,5-DIHYDROXYPHENOL
5-HYDROXYRESORCINOL
AKOS BBS-00004257
PHLOROGLUCIN
PHLOROGLUCINOL
PHLOROGLUCINOL TS
TRIHYDROXYBENZENE
?hloroglucin
1,3,5-trihydroxy-benzen
1,3,5-Trihydroxycyclohexatriene
5-Oxyresorcinol
5-Oxyresorcinolphloroglucin
Benzene, 1,3,5-trihydroxy-
Benzene, trihydroxy
benzene,trihydroxy | [EINECS(EC#)]
203-611-2 | [Molecular Formula]
C6H6O3 | [MDL Number]
MFCD00002286 | [Molecular Weight]
126.11 | [MOL File]
108-73-6.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystal | [Melting point ]
215-220 °C
| [Boiling point ]
194.21°C (rough estimate) | [bulk density]
560kg/m3 | [density ]
0.801 g/mL at 20 °C
| [vapor pressure ]
0.001Pa at 25℃ | [refractive index ]
n20/D 1.365
| [Fp ]
14 °C | [storage temp. ]
Dark Room | [solubility ]
Soluble in diethyl ether, ethanol and pyridine. | [form ]
Crystalline Powder | [pka]
pK1:8.45(0);pK2:8.88(-1) (25°C) | [color ]
White to light beige | [Water Solubility ]
11.17g/L(room temperature) | [Sensitive ]
Light Sensitive & Hygroscopic | [Merck ]
14,7328 | [BRN ]
1341907 | [InChIKey]
QCDYQQDYXPDABM-UHFFFAOYSA-N | [LogP]
0.55 at 25℃ | [CAS DataBase Reference]
108-73-6(CAS DataBase Reference) | [NIST Chemistry Reference]
1,3,5-Benzenetriol(108-73-6) | [EPA Substance Registry System]
1,3,5-Benzenetriol (108-73-6) |
| Safety Data | Back Directory | [Hazard Codes ]
F,Xi | [Risk Statements ]
R11:Highly Flammable.
R36/37/38:Irritating to eyes, respiratory system and skin .
R34:Causes burns. | [Safety Statements ]
S7:Keep container tightly closed .
S16:Keep away from sources of ignition-No smoking .
S24/25:Avoid contact with skin and eyes .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
UN 1170 3/PG 2
| [WGK Germany ]
2
| [RTECS ]
SY1050000
| [TSCA ]
Yes | [HS Code ]
29072900 | [Safety Profile]
Moderately toxic by subcutaneous and intraperitoneal routes. Mildly toxic by ingestion. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. Used in diazo-type printing and textile dyeing, in microscopy as a bone specimen decalcifier | [Toxicity]
LD50 in mice, rats (g/kg): 4.7, 4.0 i.g. (Cahen) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Tin granular-->2,4,6-TRINITROBENZOIC ACID-->Phloroglucinol Impurity 1 | [Preparation Products]
1,2,3-Trimethoxybenzene-->5,7-Dihydroxy-4-methylcoumarin-->2,4,6-TRIIODOPHLOROGLUCINOL-->CALANOLIDE A-->CIS,CIS-1,3,5-CYCLOHEXANETRIOL DIHYDRATE-->1,3,5-Cyclohexanetriol-->1-(3-ACETYL-2,4,6-TRIHYDROXYPHENYL)ETHAN-1-ONE-->9H-Xanthen-9-one, 1,3-dimethoxy-5-methyl--->2-CARBETHOXY-5,7-DIHYDROXY-4'-METHOXYISOFLAVONE-->2(4'-BROMOPHENYL)-2',4',6'-TRIHYDROXYACETOPHENONE |
| Hazard Information | Back Directory | [Description]
Phloroglucinol is a naturally occurring phenol that exhibits diverse biological activities.1 Phloroglucinol protects V79-4 Chinese hamster lung fibroblast cells from oxidative stress and inhibits lipid peroxidation by scavenging reactive oxygen species (ROS).2 It induces apoptosis in HT-29 human colon cancer cells and inhibits metastasis of BT549 and MDA-MB-231 human breast cancer cells.3,4 Phloroglucinol protects primary neurons from β-amyloid-induced dendritic spine loss in vitro and shortens the latency to find the platform in a Morris water maze test in an Alzheimer’s disease (AD) mouse model.5 Phloroglucinol has been used to stain histological plant sections and in the synthesis of numerous natural products.6,7,8 Phloroglucinol slows the frequency and decreases the amplitude of contraction in isolated rabbit and rat intestine at a concentration of 100 and 1 μM, respectively.9 Formulations containing phloroglucinol have been used as antispasmodics.10,11 | [Physical properties]
Phloroglucinol crystallizes in the anhydrous form or as the dihydrate. Recrystallization from water gives the dihydrate, which is colorless, oderless, sweet in taste, and forms rhombic crystals. The dihydrate is converted into the anhydrous form by heating at 110 ℃. Phloroglucinol sublimes at higher temperatures with partial decomposition.
| [Application]
Phloroglucinol (phlo) is a phenol derivative that shows cyctoprotective effect from oxidative damage by enhancing the activity of cellular catalase.
It can react with benzaldehyde derivatives to form phloroglucinol-based microporous polymeric organic frameworks (phlo-POF) with potential applications in ion-exchange and gas adsorption.
Phlo can also be used to prepare synthetic analogs of A-type proanthocyanidins (PACs) such as 2,8-dioxabicyclo[3.3.1]nonane derivatives by reacting with the corresponding flavylium salts. | [Definition]
ChEBI: A benzenetriol with hydroxy groups at position 1, 3 and 5. | [General Description]
Phloroglucinol is a trihydroxybenzene with antithrombotic, profibrinolytic, antimicrobial, antimalarial, cancer chemopreventive, anti-HIV and anti-leishmanial activities. Phloroglucinol (PG) is a biosynthetic precursor of the 2,4-diacetylphloroglucinol (DAPG) an antibiotic against soil-borne diseases. Phloroglucinol is a useful intermediate because it is polyfunctional. | [Flammability and Explosibility]
Notclassified | [storage]
Store at RT | [Purification Methods]
Crystallise the triol from water, and store it in the dark under nitrogen. [Beilstein 6 IV 7361.] |
| Questions And Answer | Back Directory | [Feature reaction of phloroglucinol]
Reaction of formaldehyde with phloroglucinol under alkaline conditions generates orange compound. The colorimetric assay determination of this compound at the maximum absorption wavelength of 460 nm, can be used to detect low or trace formaldehyde in textile and garment.
Since phloroglucinol may occur enol and keto tautomers, it can react with ammonia according following reaction:
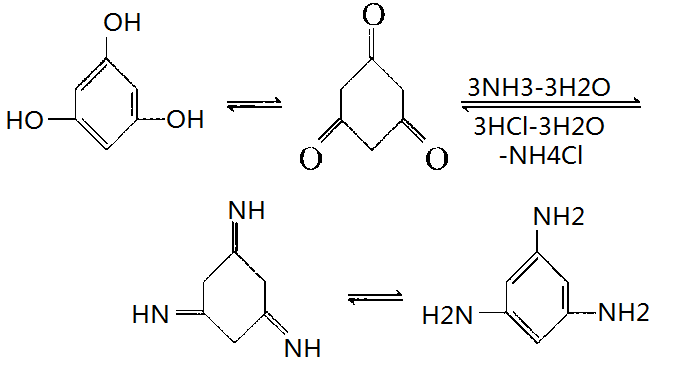
Under the influence of ammonia, 1,3,5-Benzenetriamine can be obtained by keto reaction of phloroglucinol, while this amine can also hydrolyze in aqueous acid to generate phloroglucinol. Besides phloroglucinol can also have enol reaction.
The information above is edited by Andy from Chemicalbook. | [Chemical properties]
This product is a white or light yellow crystal or crystalline powder with melting point of 218 ℃. Usually it carries two molecular crystallization water ([6099-90-7]) and converts into anhydrous at 110 ℃. It can be dissolved in 100 parts of water, 10 parts of ethanol, and 0.5 parts of pyridine and is soluble in ether. Besides It partially decomposes when sublimation, and changes its color when exposed to the light. In addition it tastes sweet. | [Uses]
Phloroglucinol can be used in verifications of antimony, arsenic, cerium, chromate, chromium, gold, iron, mercury, nitrites, osmium, palladium, tin, vanadium, vanillin and lignin, and measurement of furfural, pentose, pentosan, methanol, chloral hydrate, turpentine, Lignified cell tissues, free acid in gastric juice (HCl) and decalcified bone specimens. Besides it can also be used as biological reagent dyes. | [Production method]
2,4,6-aminobenzoic acid can be obtained by 2,4,6-trinitrobenzoic acid`s reduction of zinc particles. After its dilute solution was heated at reflux for 20h, acidified with hydrochloric acid, cooled and crystallized, phloroglucinol can be obtained with a yield of 46-53%. | [Hazards & Safety Information]
Category: Toxic substances
Toxicity grade: Moderate toxicity
Acute toxicity: oral-rat LD50: 4000 mg/kg; Oral-Mouse LD50: 4550 mg/kg
Flammability hazard characteristics: Combustible fire burning can cause smoke irritation
Storage characteristics: Ventilated, low-temperature and dry warehouse; and separately with oxidants
Extinguishing agents: Carbon dioxide, foam, sand and water mist |
|
|