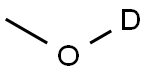(Z)-Methyl styryl ether synthesis
- Product Name:(Z)-Methyl styryl ether
- CAS Number:14371-19-8
- Molecular formula:
- Molecular Weight:0
Yield:14371-19-8 78%
Reaction Conditions:
with potassium hydroxide in dimethyl sulfoxide at 120; for 10 h;Inert atmosphere;Schlenk technique;stereoselective reaction;Reagent/catalyst;Solvent;Time;Temperature;
Steps:
General procedure for the synthesis of styryl alcohols 3-5
General procedure: In an oven dried pressure tube, to a solution of alcohols 1a-e (0.5 mmol) in 2.0 mL DMSO, finely crushed KOH (1.0 equiv.) and alkynes 2a-g (0.5 mmol) were added under an inert atmosphere. The resulting reaction mixture was stirred at 120°C for 10 h (for Z-isomer), 15 h (for E/Z-isomers), and 20 h (for E-isomers). Progression of the reaction was monitored by TLC while noticing complete consumption of alkynes, the reaction was brought to room temperature. The reaction mixture was diluted with ethyl acetate and water (10 mL × 3). The organic layer was concentrated under reduced pressure. The crude material so obtained was purified by column chromatography on silica gel mesh size 100-200 (hexane: EtOAc:90:10). The structure and purity of known starting materials were confirmed by comparison of their physical and spectral data (1H NMR, 13C NMR, and HRMS). (Z)-(2-Methoxyvinyl)benzene (3a) The product was obtained as a white oil (78%), 1H NMR (400 MHz, CDCl3) δ7.65 (d, J = 7.6 Hz, 2H), 7.36 (t, J = 7.6 Hz, 2H), 7.22 (t, J = 6.8 Hz, 1H), 6.20 (d, J = 6.8 Hz, 1H), 5.30 (d, J = 6.8 Hz, 1H), 3.84 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 148.8, 136.3, 128.5, 128.1, 125.6, 125.0, 104.9, 56.4. HRMS (ESI) [M+H]+ Calculated for [C9H10O] 135.0810 found 135.0817.
References:
Patel, Monika;Sushmita;Verma, Akhilesh Kumar [Indian Journal of Heterocyclic Chemistry,2018,vol. 28,# 1,p. 169 - 175]
865-33-8
171 suppliers
$12.50/100G
536-74-3
313 suppliers
$10.00/1g

14371-19-8
3 suppliers
inquiry
![Benzene, [(1E)-2-methoxyethenyl]-](/CAS/20180529/GIF/4110-75-2.gif)
4110-75-2
2 suppliers
inquiry

67-56-1
776 suppliers
$9.00/25ml

536-74-3
313 suppliers
$10.00/1g

14371-19-8
3 suppliers
inquiry
![Benzene, [(1E)-2-methoxyethenyl]-](/CAS/20180529/GIF/4110-75-2.gif)
4110-75-2
2 suppliers
inquiry
4009-98-7
363 suppliers
$5.00/10g
100-52-7
970 suppliers
$20.37/250g

14371-19-8
3 suppliers
inquiry
![Benzene, [(1E)-2-methoxyethenyl]-](/CAS/20180529/GIF/4110-75-2.gif)
4110-75-2
2 suppliers
inquiry