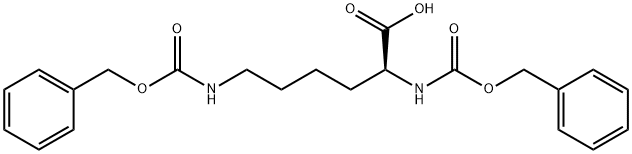
Z-Lys(Z)-OH synthesis
- Product Name:Z-Lys(Z)-OH
- CAS Number:405-39-0
- Molecular formula:C22H26N2O6
- Molecular Weight:414.45
Yield: 99%
Reaction Conditions:
with sodium carbonate in 1,4-dioxane;water; pH=Ca. 10 - 11 at 20; for 1 h;
Steps:
34 Preparation of Compound 24e
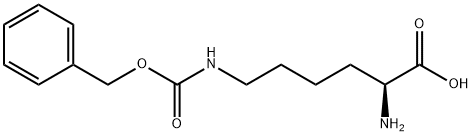
Preparation of Compound 24e A three-necked flask was loaded consecutively with H2O (40 mL), 1,4-dioxane (70 mL) and H-Lys(Z)-OH (10 g, 35.7 mmol). The mixture was stirred until complete dissolution. The pH was adjusted to about 10.5 by adding of 2 M aqueous Na2CO3. Benzyl chloroformate (6.69 g, 39.2 mmol) was added while maintaining the pH at about 10-11 by adding at the same time 2 M aqueous Na2CO3. After completing addition, the reaction mixture was stirred at 20 °C for 1 hour. Then EtOAc (50 mL) was added and pH of the resulting mixture was adjusted to 2-3 with c-HCl. The organic layer was separated and the aqueous layer was extracted with EtOAc (50 mL). The combined organic layers were washed with brine (50 mL), and dried over Na2SO4. Filtration and concentration under reduced pressure yielded the compound 24e as yellowish oil (14.7g, 99 %). 1H-NMR (400 MHz, CDC13) δ 7.33-7.27 (m, 10H), 5.07-5.04 (d, 4H), 4.08 (m, 1H), 3.09 (t, 2H), 1.51 (br s, 1H), 1.49 (bs, 1H), 1.47-1.40 (m, 4H).
References:
LEGOCHEM BIOSCIENCES, INC.;KIM, Yong, Zu;OH, Yeong, Soo;CHAE, Jeiwook;SONG, Ho, Young;CHUNG, Chul-woong;PARK, Yun, Hee;CHOI, Hyo, Jung;PARK, Kyung, Eun;KIM, Hyoungrae;KIM, Jinyeong;MIN, Ji, Young;KIM, Sung, Min;LEE, Byung, Soo;WOO, Dong, Hyun;JUN, Ji, Eun;LEE, Su, In WO2017/89890, 2017, A1 Location in patent:Page/Page column 117
![L-Lysine, N2,N6-bis[(phenylmethoxy)carbonyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/144889-39-4.gif)
144889-39-4
0 suppliers
inquiry

405-39-0
184 suppliers
$11.00/5g
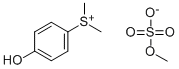
32279-04-2
34 suppliers
$70.00/5g

405-39-0
184 suppliers
$11.00/5g

![L-Lysine, N6-[(phenylmethoxy)carbonyl]-, methyl ester](/CAS/20200401/GIF/24498-31-5.gif)