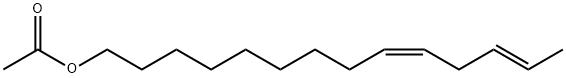
(Z,E)-9,12-TETRADECADIENYLACETATE synthesis
- Product Name:(Z,E)-9,12-TETRADECADIENYLACETATE
- CAS Number:30507-70-1
- Molecular formula:C16H28O2
- Molecular Weight:252.39
Yield: 56%
Reaction Conditions:
Stage #1:<(E)-3-Pentenyl>triphenylphosphonium-bromid with sodium hexamethyldisilazane in tetrahydrofuran at -78; for 1 h;Inert atmosphere;
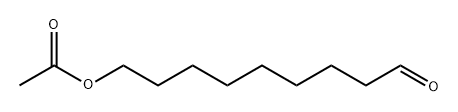
Stage #2:9-acetoxynonal in tetrahydrofuran at -78 - 20; for 24 h;Inert atmosphere;
Steps:
6 Synthesis of (9Z,12E)-tetradeca-9,12-dien-1-ol acetate
Under argon protection, (E)-3-pentenyltriphenylphosphonium bromide 4 (1.70 g, 4.13 mmol) and anhydrous tetrahydrofuran (40 mL) were added to a 100 mL Schlenk reaction flask, and stirred to dissolve. The temperature of the mixed solution was lowered to -78°C, sodium bis(trimethylsilyl)amide (2.1 mL, 2M THF solution, 4.2 mmol) was slowly added dropwise, and after the dropping, the mixture was stirred for 1 h. Then, a solution (5 mL) of 9-acetoxynonanal 7 (0.83 g, 4.13 mmol) in tetrahydrofuran was added dropwise. After dripping, the reaction solution was slowly warmed to room temperature, and the reaction was continued to be stirred for 24 hours. After the reaction was completed, saturated aqueous ammonium chloride solution (20 mL) was added dropwise under ice bath to quench the reaction. Separate the liquids, extract the aqueous phase with ether (3×30 mL), and combine the organic phases. The organic phase was washed sequentially with water (50 mL) and saturated aqueous sodium chloride solution (50 mL), and dried over anhydrous sodium sulfate. Concentrate under reduced pressure to obtain the crude product. The crude product was purified by silica gel column chromatography (petroleum ether/ethyl acetate 80:1) to obtain the pale yellow liquid compound (9Z,12E)-tetradec-9,12-diene-1-ol acetate (0.58g, yield 56%).
References:
China Agricultural University;Wang Min;Sun Xiao;Zhong Jiangchun;Yuan Chaonan;Yuan Gucheng;Yang Yuxiong;Bian Qinghua CN111205184, 2020, A Location in patent:Paragraph 0011; 0042-0044

71394-01-9
0 suppliers
inquiry

30507-70-1
65 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

30507-70-1
65 suppliers
inquiry

42521-46-0
15 suppliers
inquiry
108-24-7
0 suppliers
$14.00/250ML

30507-70-1
65 suppliers
inquiry
58257-52-6
0 suppliers
inquiry