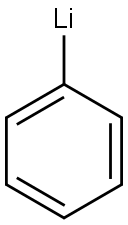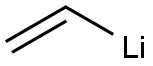
VINYL LITHIUM synthesis
- Product Name:VINYL LITHIUM
- CAS Number:917-57-7
- Molecular formula:C2H3Li
- Molecular Weight:33.99
Yield:-
Reaction Conditions:
in diethyl ether at 0; for 0.5 h;Inert atmosphere;
Steps:
1-Phenylmethyl2-methyl6- (tert-butyldiphenylsilyloxymethyl) -3-vinylpiperidine-1,2- dicarboxylate (13)
A stirred solution of tetravinyltin (4 mL, 24.5 mmol) in Et2O (10 mL)was treated with a solution of MeLi (0.98 M in Et2O, 100 mL, 98 mmol)at 0 °C, and the reaction mixture was stirred at 0 °C for 30 min. Astirred suspension of CuI (9.33 g, 49.0 mmol) in Et2O (20 mL) wastreated with a solution of vinyllithium (prepared as above) at -78 °C,and the resulting mixture was stirred at ~-78 to -35°C for 30 min. Asolution of 4 (6.1 g, 11.2 mmol) in Et2O (20 mL) was added dropwise tothe reaction mixture via a double-tipped stainless steel needle at -78°C. The reaction mixture was stirred at ~-78 to -10 °C for 1 h, and thenthe reaction was quenched with saturated aqueous NH4Cl. The aqueousmixture was diluted with CH2Cl2, and the insoluble material wasfiltered off. The organic layer was separated and the aqueous layer wasextracted with CH2Cl2 (2 × 30 mL). The organic layer and extractswere combined, dried and evaporated to give a pale yellow oil, whichwas chromatographed on silica gel (100 g, hexane:acetone =~25:1-20:1) to afford compound 13 as: Pale yellow oil;
References:
Zhou, De-Jun;Wang, Zhen-Hui;Zhang, Yan-Ru;Cui, Zheng-Guo [Journal of Chemical Research,2017,vol. 41,# 2,p. 98 - 105]
1112-56-7
125 suppliers
$75.00/5G

917-57-7
6 suppliers
inquiry
593-60-2
138 suppliers
$25.00/25g

917-57-7
6 suppliers
inquiry
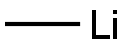

![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)