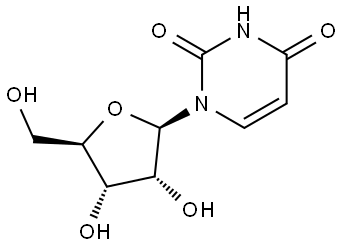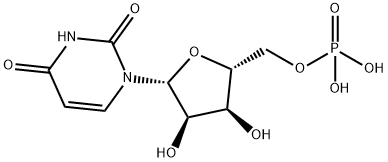
Uridine 5'-monophosphate synthesis
- Product Name:Uridine 5'-monophosphate
- CAS Number:58-97-9
- Molecular formula:C9H13N2O9P
- Molecular Weight:324.18
58-98-0
78 suppliers
inquiry

58-97-9
315 suppliers
$9.00/100mg
Yield:-
Reaction Conditions:
with human nucleotide pyrophosphatase 1;potassium chloride;2-amino-2-hydroxymethyl-1,3-propanediol;sodium chloride;calcium chloride; pH=8.5 at 37; for 3 h;
Steps:
2.8.1.2 Evaluation of resistance to of analogues 4, 8 and 18A degradation by NPP1,3
Evaluation of the activity of human NPP1 and NPP3 using para-nitrophenyl thymidine 5′-monophosphate (pnp-TMP) as a substrate, was performed as previously described.48 Next, the percentage of hydrolysis of analogues 4, 8 and 18A by NPP1,3 was evaluated as follows: 56.15 μg or 57.78 μg of human NPP1 or NPP3 extract, respectively, was added to 0.575 mL the incubation mixture (1 mM CaCl2, 200 mM NaCl, 10 mM KCl and 100 mM Tris, pH 8.5) and pre-incubated at 37 °C for 3 min. Reaction was initiated by the addition of 0.015 mL of 4 mM of each analogue (4, 8 or 18A). The reaction was stopped after 2 h or 3 h NPP1 or NPP3, respectively, by transferring a 0.1 mL aliquot from the reaction mixture to 0.350 mL ice-cold 1 M perchloric acid. These samples were centrifuged for 1 min at 10,000×g. Supernatants were neutralized with 170 μL 2 M KOH in 4 °C and centrifuged 1 min at 10,000×g. The reaction mixture was filtered and freezed-dried. The hydrolysis rates of the analogues 4, 8 and 18A by NPP1 or NPP3 were determined by measuring the change in the integration of the HPLC peaks for each analogue over time versus control. The percentage of compound degradation was calculated versus control, to take into consideration the degradation of the compounds due to the addition of acid to stop the enzymatic reaction. Therefore, each of the samples was compared to a control which was transferred to acid, but to which no enzyme was added. The percentage of degradation was calculated from the area under the curve of the nucleoside monophosphate peak, after subtraction of the control, which is the amount of the nucleoside monophosphate peak formed due to chemical acidic hydrolysis.
References:
Ginsburg-Shmuel, Tamar;Haas, Michael;Grbic, Djordje;Arguin, Guillaume;Nadel, Yael;Gendron, Fernand-Pierre;Reiser, Georg;Fischer, Bilha [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 18,p. 5483 - 5495]
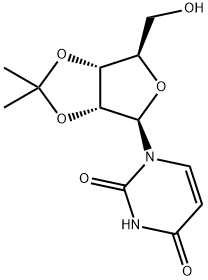
362-43-6
126 suppliers
$38.00/5g

58-97-9
315 suppliers
$9.00/100mg
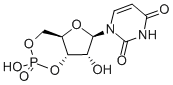
4004-57-3
2 suppliers
inquiry

58-97-9
315 suppliers
$9.00/100mg
![(2S,3S,4R,5R,6R)-6-[[[(2S,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-hydroxy-phosphoryl]oxy-hydroxy-phosphoryl]oxy-3,4,5-trihydroxy-oxane-2-carboxylic](/CAS/20210111/GIF/2616-64-0.gif)
![[5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[hydroxy-(3,4,5-trihydroxyoxan-2-yl)oxy-phosphoryl]oxy-phosphinic acid](/CAS/GIF/3616-06-6.gif)