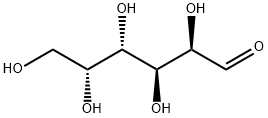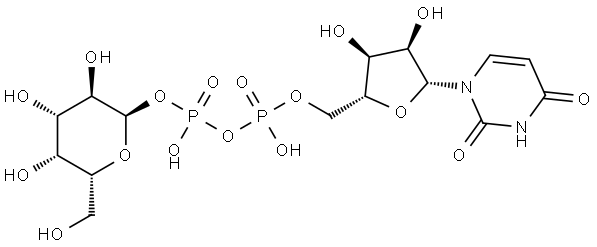
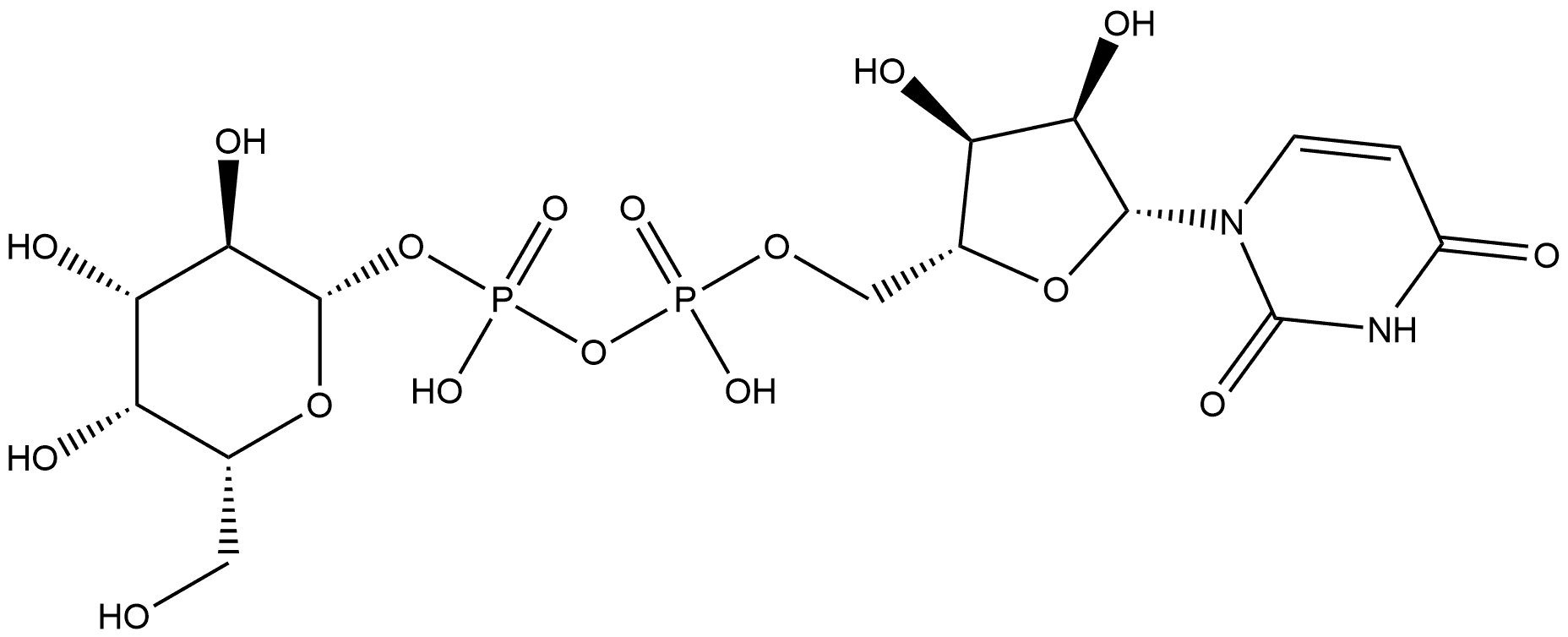
UDP-D-galactose synthesis
- Product Name:UDP-D-galactose
- CAS Number:2956-16-3
- Molecular formula:C15H24N2O17P2
- Molecular Weight:566.3
10257-28-0
77 suppliers
$138.00/100g
63-39-8
140 suppliers
$50.00/10mg

2956-16-3
57 suppliers
inquiry
Yield:2956-16-3 86%
Reaction Conditions:
with Pasteurella multocida inorganic pyrophosphatase;Bifidobacterium longumuridine 5'-diphosphate-sugarpyrophosphorylase;Echerichia coli galactokinase;adenosine-5'-triphosphate;magnesium chloride in aq. buffer at 37; pH=8; for 24 h;Enzymatic reaction;Reagent/catalyst;
Steps:
4 Uridine 5′-diphospho-α-D-galactopyranoside (UDP-Gal, T6-16)
Monosaccharides and derivatives (30-100 mg, 1.0 eq.), ATP (1.2 eq.), and UTP (1.3 eq.) were dissolved in water in a 15 mL centrifuge tube containing Tris-HCl buffer (100 mM, pH 8.0) and MgCl2 (10 mM). After the addition of appropriate amount of NahK_ATCC15697, EcGalK, or SpGalK (1.3-4.5 mg), BLUSP (1.0-2.5 mg), and PmPpA (1.5-2.5 mg), millipore water was added to bring the total volume of the reaction mixture to 10 mL. The reaction was carried out by incubating the solution in an isotherm incubator for 24 hr at 37° C. with gentle shaking or without shaking. In the synthesis of UDP-Glc, commercially available Glc-1-P (55.2 mg), UTP (1.2 eq.), Tris-HCl buffer (100 mM, pH 8.0), and MgCl2 (10 mM) were used along with BLUSP (1 mg) and PmPpA (1.5 mg). The reaction was left for 2 hr at 37° C. in isotherm with gentle shaking. Product formation was monitored by TLC (EtOAc:MeOH:H2O:AcOH=5:3:3:0.3 by volume) with p-anisaldehyde sugar staining. The reaction was terminated by adding the same volume of ice-cold ethanol and incubating at 4° C. for 30 min followed by centrifugation remove the enzymes. The supernatant was collected and concentrated and passed through a BioGel P-2 gel filtration column to afford the product. Silica gel column purification (EtOAc:MeOH:H2O=7:3:2) was applied when necessary to achieve further purification.135 mg. Yield, 86%; white foam. 1H NMR (600 MHz, D2O) δ 7.93 (d, J=8.4 Hz, 1H), 5.97-5.95 (m, 2H), 5.63 (dd, J=7.2, 3.6 Hz, 1H), 4.37-4.35 (m, 2H), 4.28-4.18 (m, 3H), 4.16 (t, J=6 Hz, 1H), 4.02 (d, J=3 Hz, 1H), 3.90 (dd, J=10.2, 3.6 Hz, 1H), 3.80 (dt, J=10.2, 3.3 Hz, 1H), 3.76-3.71 (m, 2H). 13C NMR (150 MHz, D2O) δ 166.39, 151.96, 141.78, 102.80, 96.01 (d, J=6.6 Hz), 88.65, 83.32 (d, J=8.9 Hz), 73.93, 72.11, 69.78, 69.43, 69.24, 68.50 (d, J=7.8 Hz), 65.15 (d, J=5.0 Hz), 61.16. HRMS (ESI) m/z calcd for C15H24N2O17P2 (M-H) 565.0472, found 565.0453.
References:
US2014/235575,2014,A1 Location in patent:Paragraph 0323; 0324; 0325
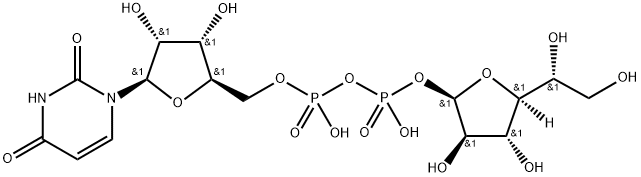
26097-62-1
0 suppliers
inquiry

2956-16-3
57 suppliers
inquiry
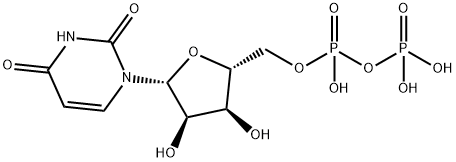
58-98-0
78 suppliers
inquiry
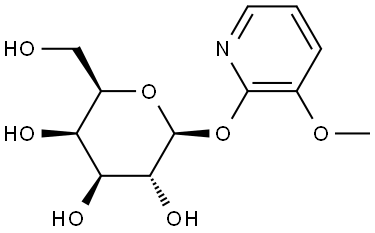
170428-70-3
1 suppliers
inquiry

2956-16-3
57 suppliers
inquiry
7349-77-1
0 suppliers
inquiry

10257-28-0
77 suppliers
$138.00/100g

58-98-0
78 suppliers
inquiry

2956-16-3
57 suppliers
inquiry

7349-77-1
0 suppliers
inquiry