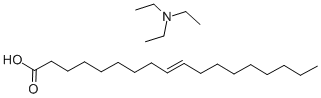
TRIETHYLAMINE OLEATE synthesis
- Product Name:TRIETHYLAMINE OLEATE
- CAS Number:10103-17-0
- Molecular formula:C24H49NO2
- Molecular Weight:383.65

10103-17-0
6 suppliers
inquiry
13552-09-5
11 suppliers
$283.00/25mg

541-41-3
6 suppliers
$21.00/5g
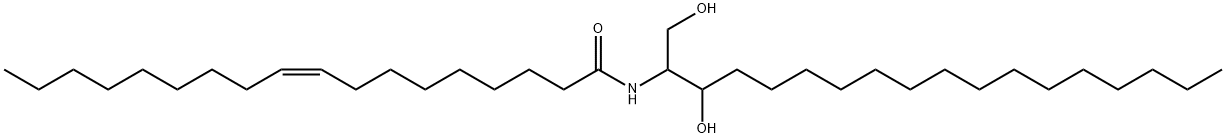
54422-45-6
2 suppliers
inquiry
Yield:54422-45-6 75%
Reaction Conditions:
in tetrahydrofuran;
Steps:
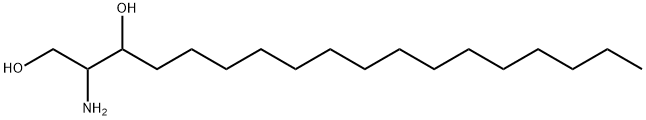
2 Preparation of 2-oleoylamino-1,3-octadecanediol
EXAMPLE 2 Preparation of 2-oleoylamino-1,3-octadecanediol 3 ml of ethyl chloroformate (31 mmol) in 5 ml of tetrahydrofuran are solubilized in a round-bottomed flask under an inert atmosphere; this solution being cooled to -15° C., 11.3 g of triethylamine oleate (29.5 mmol) previously solubilized in 10 ml of tetrahydrofuran, are added dropwise. After stirring for 2 hours at room temperature, the reaction mixture is slowly poured, under an inert atmosphere, into 8.9 g of (D,L)-2-amino-1,3-octadecanediol prepared in the 1st stage of Example 1 (29.5 mmol) solubilized in 40 ml of tetrahydrofuran at 30° C. After stirring for 1 hour at 30° C., under an inert atmosphere, (thin layer chromatography on silica gel, eluent methylene chloride/methanol/ammonium hydroxide 15/3.5/0.6), the reaction mixture is washed with water and then evaporated to dryness under vacuum. The crude product obtained is chromatographed on a silica column (eluent: ethyl acetate/heptane 5/3) and 12.5 g of white crystals (yield 75%) are obtained after evaporation of the solution solvent under vacuum.
References:
US5618523,1997,A