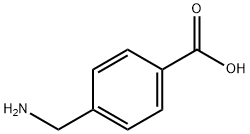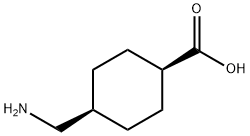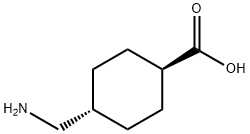
Tranexamic Acid synthesis
- Product Name:Tranexamic Acid
- CAS Number:1197-18-8
- Molecular formula:C8H15NO2
- Molecular Weight:157.21

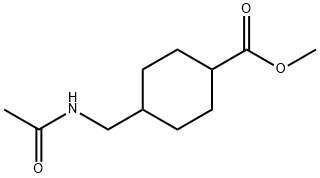
51782-10-6
1 suppliers
inquiry

1197-18-8
840 suppliers
$10.00/5g
Yield:1197-18-8 85.11%
Reaction Conditions:
with barium hydroxide octahydrate;hydrogen in ethanol;water at 250; under 7500.75 - 30003 Torr; for 7.5 h;Pressure;Temperature;
Steps:
5 Preparation of trans-4-aminomethylcyclohexylformic acid
To a 1 L hydrogenation kettle, methyl 4-acetamidomethylcyclohexylcarboxylate (28. 00 g, 0.13 mol), 100 mL of ethanol, 200 mL of water,Barium hydroxide octahydrate (95. 82g, 0.30mol), shut down the reaction dad, pass the IMPa hydrogen to replace the air 3 times (or replace with nitrogen)And then pass 1MPa of hydrogen leak detection, if not leak, the pressure transferred to 4MPa, stir to raise the temperature to 250 ° C reaction 7. 5 hours,Stop the reaction, open the kettle, the reaction solution poured out, placed in a reaction flask, add water 200mL, heated to 70 ° C,Neutralized with carbon dioxide to pH 3. 3, barium carbonate was removed by filtration, the filtrate was adjusted to pH 3 with sulfuric acid, allowed to stand for 3 hours, filtered, washed with water,Add 5 g of activated charcoal decolorization, filtration, the filtrate sprinkled to 50mL, then add ethanol 50mL crystallization, cooling to 10 ° C, filtration, crystallization with ethanol washing, drying, white solid trans-4-aminomethylcyclohexylformic acid, Mass of 17.37g, mp: 384-388 ° (decomposition), molar yield of 85.11% By liquid phase detection, trans-body content of 99.6910%
References:
CN104151183,2016,B Location in patent:Paragraph 0051-0053
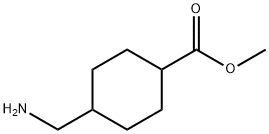
23199-14-6
27 suppliers
$279.23/1gm:

1197-18-8
840 suppliers
$10.00/5g
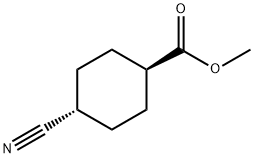
19145-95-0
17 suppliers
inquiry

1197-18-8
840 suppliers
$10.00/5g
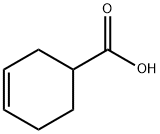
4771-80-6
281 suppliers
$5.00/10g

1197-18-8
840 suppliers
$10.00/5g