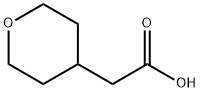
Tetrahydropyranyl-4-acetic acid synthesis
- Product Name:Tetrahydropyranyl-4-acetic acid
- CAS Number:85064-61-5
- Molecular formula:C7H12O3
- Molecular Weight:144.17
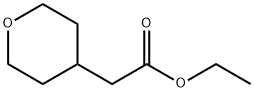
103260-44-2
110 suppliers
$10.00/100mg

85064-61-5
156 suppliers
$6.00/100mg
Yield: 94%
Reaction Conditions:
with water;sodium hydroxide in methanol at 0 - 20; for 15 h;
Steps:
3.3 Step 3: Benzyl 2-(tetrahydro-2H-pyran-4-yl)acetate
A solution of sodium hydroxide (15.9 g, 44.06 mmol) in water (150 ml) was added dropwise to a solution of ethyl 2-(tetrahydro-2H-pyran-4-yl)acetate (15 g, 8.81 mmol) in methanol (150 ml) at a temperature of 0 °C or below, and the mixture was stirred at room temperature for 15 hr. The solvent of the reaction mixture was removed by distillation under reduced pressure, and the water layer was adjusted to pH 3 by the addition of 1 M hydrochloric acid and was extracted with ethyl acetate. The organic layer was washed with saturated brine and was dried over anhydrous sodium sulfate, and the filtrate was concentrated under reduced pressure to give a corresponding acid as a colorless solid (12.0 g, 94%). This compound was used in the next step without further purification. Benzyl bromide (14.3 g, 83.33 mmol) was added dropwise to a suspension of this compound (10.0 g, 69.44 mmol) and anhydrous potassium carbonate (28.8 g, 208.3 mmol) in acetonitrile (100 ml) at room temperature, and the mixture was refluxed for 48 hr. The solvent of the mixture was removed by distillation under reduced pressure, the residue was diluted with water and was extracted with dichloromethane, and the organic layer was dried over anhydrous sodium sulfate. The filtrate was concentrated under reduced pressure, and the residue was chromatographed on silica gel column (hexane:ethyl acetate = 9:1) to give the tile compound as an oil (12.0 g, yield 75%). 1H-NMR (400 MHz, CDCl3): δ (ppm) 7.39-7.34 (m, 5H), 5.12 (s, 2H), 3.95-3.92 (m, 2H), 3.42-3.36 (m, 2H), 2.30 (d, J = 7.2 Hz, 2H), 2.09-1.99 (m, 1H) 1.64-1.61 (m, 2H), 1.39-1.29 (m, 2H); MS (ESI): m/z 234 (M+).
References:
Meiji Seika Pharma Co., Ltd.;MORINAKA, Akihiro;MAEBASHI, Kazunori;IDA, Takashi;HIKIDA, Muneo;YAMADA, Mototsugu;ABE, Takao EP2737900, 2014, A1 Location in patent:Paragraph 0114; 0117-0118
156002-64-1
76 suppliers
$10.00/250mg

85064-61-5
156 suppliers
$6.00/100mg

29943-42-8
430 suppliers
$5.00/1g
867-13-0
483 suppliers
$10.00/1g

85064-61-5
156 suppliers
$6.00/100mg

29943-42-8
430 suppliers
$5.00/1g

85064-61-5
156 suppliers
$6.00/100mg
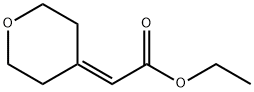
130312-00-4
86 suppliers
$40.00/250 mg

85064-61-5
156 suppliers
$6.00/100mg