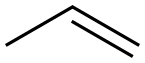
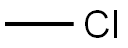Tetrachloroethylene synthesis
- Product Name:Tetrachloroethylene
- CAS Number:127-18-4
- Molecular formula:C2Cl4
- Molecular Weight:165.83
The industrial processes for production of tetrachloroethylene include threetechnical routes:
(1) chlorination of trichloroethylene and followed by dehydrochlorination.
(2) oxychlorination of ethylene.
(3) chlorination andpyrolysis of light hydrocarbon.
In China, the chlorination of trichloroethylene and dehydrochlorination process is mainly used for production of tetrachloroethylene, while the other two processes are widely used in other countries.
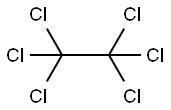
67-72-1
282 suppliers
$11.00/25g

127-18-4
765 suppliers
$15.00/5G
Yield:127-18-4 97%
Reaction Conditions:
in tetrachloromethane at 300; for 0.000555556 h;Flow reactor;Pyrolysis;Temperature;
Steps:
3
After the mixture of perchloroethane and carbon tetrachloride by mass ratio of 1: 0.5,With the measurement of Li to 2ml / min flow rate into the diameter 2. Ocm,Length 2 m quartz glass reactor,At the same time, the reaction was carried out in a quartz glass reactor at a flow rate of 0.3 ml / min,The temperature of the quartz glass reactor was controlled at 300 ° C,The contact time of the raw material with the reactor was 2 s through flow control,The pyrolysis gas obtained at the outlet of the quartz glass reactor is condensed,The purity of 99% tetrachlorethylene was obtained by distillation. The sample was sampled from the outlet of the quartz glass reactor. The conversion of perchlorethylene was 98%, the selectivity of tetrachloroethylene was 99% and the reaction yield was 97%.
References:
CN104292069,2016,B Location in patent:Paragraph 0032; 0033
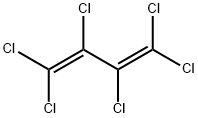
87-68-3
156 suppliers
$10.00/1g

127-18-4
765 suppliers
$15.00/5G

67-72-1
282 suppliers
$11.00/25g
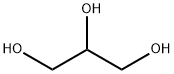
56-81-5
1699 suppliers
$5.00/25g

127-18-4
765 suppliers
$15.00/5G
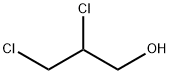
616-23-9
149 suppliers
$42.63/5g
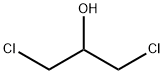
96-23-1
293 suppliers
$10.00/1g
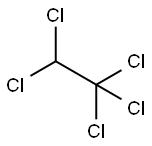
76-01-7
68 suppliers
$57.20/5ml