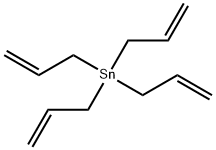
Tetraallyltin synthesis
- Product Name:Tetraallyltin
- CAS Number:7393-43-3
- Molecular formula:C12H20Sn
- Molecular Weight:283
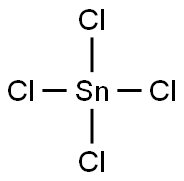
7646-78-8
314 suppliers
$11.00/5g

1730-25-2
202 suppliers
$53.20/100ml

7393-43-3
92 suppliers
$52.42/1G
Yield: 89%
Reaction Conditions:
in diethyl ether at -78 - 20; for 2 h;Inert atmosphere;
Steps:
4.1.1 Tetraallylstannane
In a 1 L round-bottomed flask equipped with a magnetic stirrer, dropping funnel, and an argon inlet/outlet an ethereal solution of allylmagnesium bromide (470 mL, 0.75 M, 352 mmol) was placed (under argon). The solution was cooled to -78 °C and, while vigorously stirred, tin(IV) chloride (8.60 mL, 74 mmol) was added dropwise (within ca. 10 min). After the addition was completed, the mixture was allowed to warm to rt (within 1 h) and stirring was continued for 1 h (1.5 h in temp above 0 °C in total). The mixture was then cooled again to -78 °C and satd aq NH4Cl (94 mL) was added dropwise. The mixture was allowed to warm to rt. The solution was decanted from the semi-crystalline mass, dried, and filtered through Celite. The reaction vessel, the drying agent, and the Celite were washed consecutively with ether (50 mL). The ethereal solutions were combined and the solvent was evaporated. The residue (20.5 g) was distilled collecting fraction at 75 °C/2.5 mmHg. The title product was obtained (colorless liquid, 18.57 g, 89% yield). 1H NMR in agreement with that previously reported.29
References:
Michalak, Karol;Wicha, Jerzy [Tetrahedron,2014,vol. 70,# 34,p. 5073 - 5081]

7646-78-8
314 suppliers
$11.00/5g
7439-95-4
0 suppliers
$11.60/0.9g
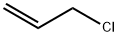
107-05-1
420 suppliers
$16.80/100ML

7393-43-3
92 suppliers
$52.42/1G
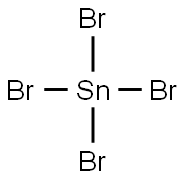
7789-67-5
59 suppliers
$57.00/25g

1730-25-2
202 suppliers
$53.20/100ml

7393-43-3
92 suppliers
$52.42/1G
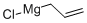
2622-05-1
156 suppliers
$40.00/500mg

7646-78-8
314 suppliers
$11.00/5g

7393-43-3
92 suppliers
$52.42/1G

7440-31-5
325 suppliers
$15.00/100G
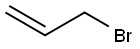
106-95-6
433 suppliers
$10.00/5g

7393-43-3
92 suppliers
$52.42/1G