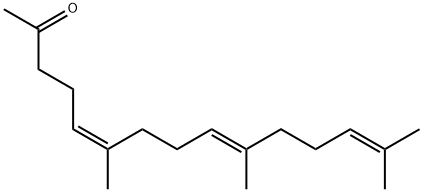
Teprenone Impurity 20 synthesis
- Product Name:Teprenone Impurity 20
- CAS Number:1117-51-7
- Molecular formula:C18H30O
- Molecular Weight:262.43
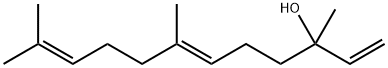
40716-66-3
199 suppliers
$6.00/5g
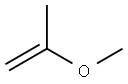
116-11-0
279 suppliers
$10.00/5g

1117-51-7
11 suppliers
inquiry
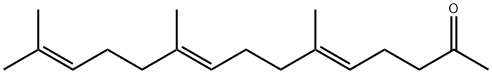
1117-52-8
122 suppliers
$6.00/1g
Yield:-
Reaction Conditions:
Stage #1: (E)-3,7,11-trimethyl-1,6,10-dodecatrien-3-ol;2-Methoxypropene;phosphoric acid in acetone at 160; for 16 h;
Stage #2: with sodium acetate in acetone at 25; for 0.5 h;Product distribution / selectivity;
Steps:
1; 2; 3
A 1.0 liter stainless steel batch reactor (Medimex - High Pressure) with electrical heating and water cooling system in the jacket, a pressure sensor and stainless steel propeller was loaded with a mixture of 1.2885g of a solution of 18w% H3PO4 in acetone (2.37 mMol, 0.3 Mol%) and 179.5g of E-nerolidol (0.790 MoI). 225g of isopropenylmethyl ether (IPM, 3.01 MoI, 3.81 equivalent) were added. Under stirring (500 rpm) the mixture was heated to 1600C within two hours. After 16 hours total reaction time the reaction mixture was cooled to 25°C, drawn from the reactor under reduced pressure and stirred with 2g of sodium acetate for 30 minutes. After filtration over a 5μm Teflon membrane the low- boilers were removed in two steps (at lOmbar and 0.05mbar on pump) in a Rota vapor at 400C. The crude product (yield 93.1%, selectivity 0.94) was distilled in a 500ml two- necked round-bottomed flask with PT 100, magnetic stirrer, Liebig condenser, Anschtz Thiele separator, cold trap and high- vacuum pump in an oil bath. At a bath temperature of 1400C, an inner temperature of 125 - 127°C, a head temperature of 118 - 122°C and an absolute pressure of 0.03 - 0.02 mbar (on the pump) a mixture OEIE- and (5Z,9E)- farnesyl acetone was obtained in a yield of 86%. A pre-fraction with a mixture of EIE- and (5Z,9£)-farnesyl acetone was obtained in a yield of 3.7% at 67 - 1400C, 44.5 - 123°C, 27.3 - 111°C, respectively, and 0.03mbar (on pump). Total yield of the main- and pre- fraction 89.7%, selectivity 0.9). The .E/.E-farnesyl acetone was separated from the (5Z/9E)- farnesyl acetone by distillation. The product was characterized by 1H and 13C-NMR spectroscopy. The purity was determined by gas chromatography.E/E-FA 300 MHz 13C-NMR (CDCl3): δ (ppm, TMS) = 208.8, 136.4, 135.0, 131.3, 124.4, 124.0, 122.5, 43.8, 39.7, 39.6, 29.9, 26.8, 26.5, 25.5, 22.5, 17.7, 16.0 (2C).; Example 2In the same manner as described in Example 1 starting from 199g .E-nerolidol, 1.38g catalyst solution and 3.15 equivalents of IPM there was obtained a mixture of E/ E- and (5Z,9£)-farnesyl acetone in a yield of 89.8%, selectivity 0.90 (crude) and of 87.4% (Total of main- and pre-fraction, after separation of the low- and high-boilers) with a selectivity of θ.87.; Example 3In the same manner as described in Example 1 staring from 224.4g .E-nerolidol, 1.56g of catalyst solution and 2.5 equivalents of IPM there was obtained a mixture OEIE- and (5Z,9£)-farnesyl acetone in a yield of 88.2%, selectivity 0.88 (crude) and of 86.1% (Total of main- and pre-fraction, after separation of the low- and high-boilers) with a selectivity of θ.86.
References:
WO2009/19132,2009,A1 Location in patent:Page/Page column 4-5
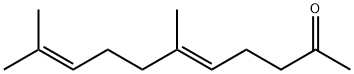
3796-70-1
176 suppliers
$6.00/5g
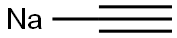
1066-26-8
98 suppliers
$48.00/50g

1117-51-7
11 suppliers
inquiry

1117-52-8
122 suppliers
$6.00/1g

78-70-6
625 suppliers
$26.00/25mL

1117-51-7
11 suppliers
inquiry
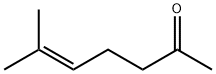
110-93-0
313 suppliers
$8.00/25g

1117-51-7
11 suppliers
inquiry
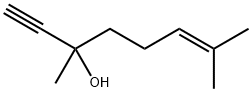
29171-20-8
60 suppliers
$16.00/5g

1117-51-7
11 suppliers
inquiry