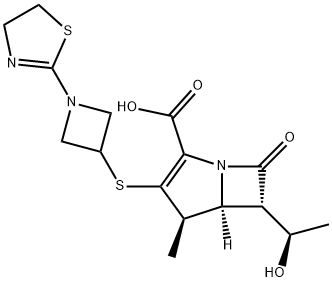
Tebipenem synthesis
- Product Name:Tebipenem
- CAS Number:161715-21-5
- Molecular formula:C16H21N3O4S2
- Molecular Weight:383.49
![4-Nitrobenzyl(1R,5S,6S)-6-[(R)-1-hydroxyethyl]-1-Methyl-2-[1-(1,3-thiazolin-2-yl)azetidin -3-yl]thio-1-carbapen-2-eM-3-carboxylate](/CAS/GIF/161715-20-4.gif)
161715-20-4
36 suppliers
inquiry

161715-21-5
82 suppliers
inquiry
Yield: 94.5%
Reaction Conditions:
with hydrogen in water;butan-1-ol at 20; under 15001.5 Torr; for 4 h;Solvent;Pressure;Reagent/catalyst;
Steps:
1
The compound (II) (R is p-nitrobenzyl) (15.0 g, 29.Ommo 1) was added 200 mL of n-butanol, 200 mL of water, stirred to dissolve, and 8.00 g of Raney Ni was added and hydrogenated at 20 ° C under 2.0 MPa hydrogen pressure for 4 h. The filter cake was washed with 15 mL of water The filtrate was adjusted to pH 6.5 with 4-dimethylaminopyridine and then washed with 150 mL of ethyl acetate. After cooling to 0 ° C, 700 mL of acetone was added dropwise, stirred for 10 min and then 700 mL of acetone was added dropwise, stirred for 1 h, To give telbemidine as a white solid 12.5 g, yield 94.5%, purity 99.6%.
References:
Shanghai Institute of Pharmaceutical Industry;Luo, ying;Liu, xiangkui;Shen, yuhui;Zhu, xueyan;Yuan, zhedong CN102757430, 2016, B Location in patent:Paragraph 0040; 0041
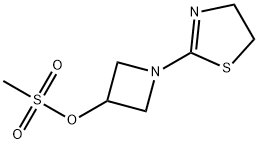
179337-62-3
7 suppliers
inquiry

161715-21-5
82 suppliers
inquiry
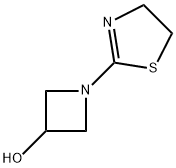
161715-27-1
32 suppliers
inquiry

161715-21-5
82 suppliers
inquiry
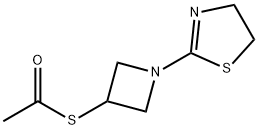
161715-28-2
8 suppliers
inquiry

161715-21-5
82 suppliers
inquiry