Tazarotene synthesis
- Product Name:Tazarotene
- CAS Number:118292-40-3
- Molecular formula:C21H21NO2S
- Molecular Weight:351.46
118292-06-1
152 suppliers
$9.00/100mg
49608-01-7
271 suppliers
$7.00/5g

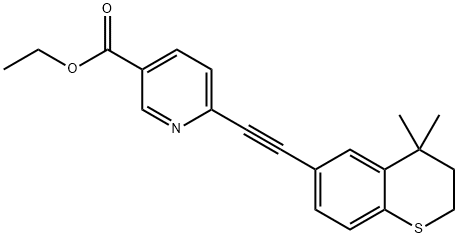
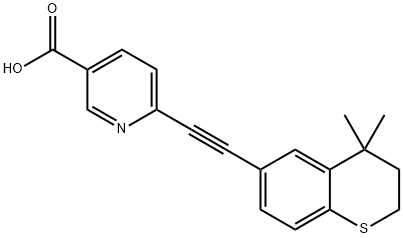
118292-40-3
307 suppliers
$25.00/10mg
Yield:118292-40-3 60%
Reaction Conditions:
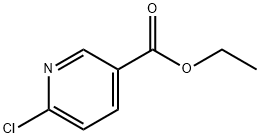
Stage #1:6-chloro-3-pyridinecarboxylic acid ethyl ester with potassium carbonate;triphenylphosphine;5%-palladium/activated carbon in toluene at 45 - 50;
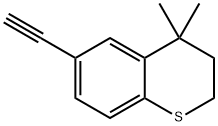
Stage #2:(4,4-dimethylthiochroman-6-yl)acetylene;copper(l) iodide in toluene at 45 - 115;Product distribution / selectivity;
Steps:
6
Charged ethyl 6-chloronicotinate (10.0 gm) in a R.B. Flask arranged with reflux condenser on oil bath, containing toluene (100 ml). Stirred and charged potassium carbonate (30.0 gm), triphenylphosphine (4.0 gm) and 5% Pd/C (4.0 gm) to the reaction solution. Gradually raised the temperature to 45-50 C and maintained under stirring at 45 - 50°C for 2 hours. Charged 6-ethynyl-4,4-dimethylthiochromane (8 gm) and Copper iodide (0.05 gm) at 45-50°C. After charging, the reaction temperature was raised to 105- 115°C and maintained under stirring at 105-1150C temperature for 10 hours. After completion, the reaction mass was cooled to 25-300C and filtered the catalyst Pd/C. To the filtrate charged water (300 ml) and stirred for 30 min. Separated the organic layer and the aqueous layer was extracted with toluene (100 ml). Separated the organic layer and combined all organic layers. Concentrated the solvent under reduced pressure to get residual mass. Charged hexane (25 ml) to the residual mass and stirred at 25-300C for 1.0 hour. Cooled the reaction to -10 to 5°C and maintained for 1.0 hour. Filtered the solid Tazarotene and dried in oven till constant weight. Weight of Tazarotene = 8.3 gm % Yield = 60.0 %.
References:
INDOCO REMEDIES LIMITED WO2009/116075, 2009, A2 Location in patent:Page/Page column 12

118292-06-1
152 suppliers
$9.00/100mg

118292-40-3
307 suppliers
$25.00/10mg

64-17-5
726 suppliers
$10.00/50g
118292-41-4
76 suppliers
$85.00/5mg

118292-40-3
307 suppliers
$25.00/10mg
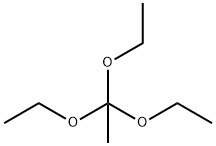
78-39-7
352 suppliers
$9.00/1g

118292-41-4
76 suppliers
$85.00/5mg

118292-40-3
307 suppliers
$25.00/10mg
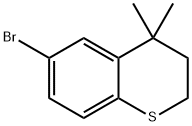
112110-44-8
56 suppliers
$17.00/250mg

118292-40-3
307 suppliers
$25.00/10mg