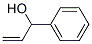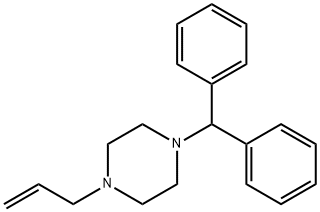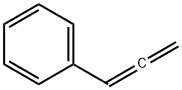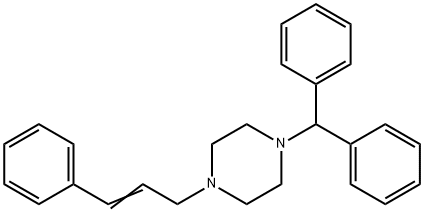
Stugeron synthesis
- Product Name:Stugeron
- CAS Number:298-57-7
- Molecular formula:C26H28N2
- Molecular Weight:368.51
Yield:298-57-7 90%
Reaction Conditions:
in o-xylene at 150; for 30 h;Inert atmosphere;Sealed tube;
Steps:
2.4. Catalytic amination reactions
General procedure: Dried pressure tube was charged with magnetic stir bar and50 mg of PdSiO2 catalysts (1 mol% with respect to amine). Then,1.0 mL o-xylene was added, followed by the addition of 0.5 mmolof amine and 1 mmol of benzyl alcohol. The pressure tube wasflushed with argon was closed with screw cap. Then it was placedin the preheated aluminum block and reaction was allowed to progressfor 30 h at 150 °C. After completion of the reaction, pressuretube was removed from aluminum block and cooled down to roomtemperature. The catalyst was filtered out by ciliate and reactionproducts were analyzed by GC-MS and the corresponding amineswere purified by column chromatography. The yields of selectedamines were determined by GC analysis using n-hexadecane asstandard. For this purpose, after completion of the reaction, nhexadecane(100 mL) as standard was added to the reaction pressuretube and the reaction products were diluted with ethyl acetate followed by filtration using plug of silica and then subjected GCanalysis.
References:
Alshammari, Ahmad S.;Natte, Kishore;Kalevaru, Narayana V.;Bagabas, Abdulaziz;Jagadeesh, Rajenahally V. [Journal of Catalysis,2020,vol. 382,p. 141 - 149]
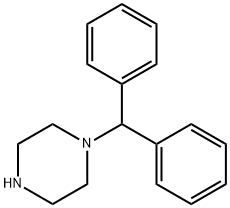
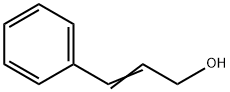
841-77-0
347 suppliers
$10.00/5g
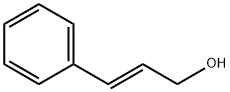
4407-36-7
79 suppliers
$18.00/25g

298-57-7
429 suppliers
$5.00/25mg