SPIROTETRAMAT synthesis
- Product Name:SPIROTETRAMAT
- CAS Number:203313-25-1
- Molecular formula:C21H27NO5
- Molecular Weight:373.44
![cis-3-(2,5-Dimethylphenyl)-4-hydroxy-8-methoxy-1-azaspiro[4.5]dec-3-en-2-one](/CAS/20180703/GIF/203312-38-3.gif)
203312-38-3
19 suppliers
$100.00/10mg

541-41-3
0 suppliers
$19.39/5g

203313-25-1
0 suppliers
$38.00/25mg
Yield:203313-25-1 85%
Reaction Conditions:
with triethylamine in dichloromethane at 20 - 30;
Steps:
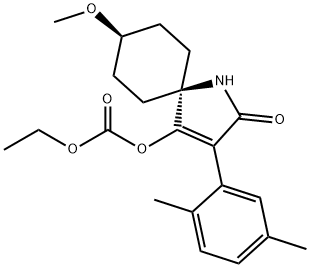
cis-3-(2,5-Dimethylphenyl)- 8 -methoxy-2-oxo-1-azaspiro[4.5]dec-3-en-4-yl ethyl carbonate (10)
Compound 9 (300 g, 1 mol) was added to 1500 mL of dichloromethane, and the mixture was stirred for 10 min. Triethylamine (120 g, 1.2 mol) was then added slowly at 20-25°C, ethyl chloroformate (135 g, 1.2 mo l ) was added over a period of 0.5-1 h, and the mixture was stirred at 20-30°C until the conversion of 9 was more than 99% (HPLC). The mixture was treated with water (650 g) and concentrated hydrochloric acid (40 g) and stirred for 20 min. The organic phase was separated and evaporated under atmospheric pressure at 75°C. Petroleum ether (1000 mL) was added to the residue, the mixture was refluxed for 30 min, cooled down to 30°C at a rate of 15-20 deg per hour, and the precipitate was filtered off to obtain compound 10 (320 g, 85%) as white powder, mp 287- 290°C [15]. 1H NMR spectrum (CDCl3), δ, ppm: 1.10 t (3H, CH3, J = 4.0 Hz), 1.36-1.47 m (2H, CH2), 1.75 d (2H, CH2, J = 12.0 Hz), 1.93 t (2H, CH2, J = 12.0 Hz), 2.21-2.25 m (3H, CH3), 2.26 d (2H, CH2, J = 4.4 Hz), 2.29 s (3H, CH3), 3.21-3.28 m (1H, CH), 3.21-3.28 m (1H, CH), 3.39 s (3H, CH3), 4.02 d.d (2H, CH2, J = 4.0, 8.0 Hz), 6.75 s (1H, CH), 6.99 s (1H, CH), 7.04 d (1H, CH, J = 12.0 Hz), 7.11 d (1H, CH, J = 4.0 Hz). 13C NMR spectrum (CDCl3), δC, ppm: 13.72, 19.21, 20.89, 28.40, 31.65, 55.85, 60.25, 65.74, 121.43, 127.79, 129.48, 129.91, 130.20, 133.96, 134.98, 149.87, 164.76, 170.01.
References:
Mao, Longfei;Wang, Jiahao;Xu, Guiqing;Zhou, Yingjie [Russian Journal of Organic Chemistry,2020,vol. 56,# 10,p. 1775 - 1778] Location in patent:supporting information
13482-23-0
208 suppliers
$20.00/250mg

203313-25-1
0 suppliers
$38.00/25mg