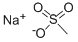
SODIUM METHANESULFONATE synthesis
- Product Name:SODIUM METHANESULFONATE
- CAS Number:2386-57-4
- Molecular formula:CH3NaO3S
- Molecular Weight:118.09
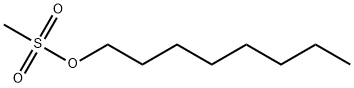
1912-32-9
142 suppliers
$6.00/1g
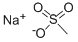
2386-57-4
233 suppliers
$13.00/25g
Yield:-
Reaction Conditions:
with sodium hydroxide;Phthalic acid dibutyl ester;water at 90; for 0.25 - 3 h;Conversion of starting material;
Steps:
6
Methyl methanesulfonate (MMS), methyl para-toluenesulfonate (MTS), butyl methanesulfonate (BMS), or butyl para-toluenesulfonate (BTS) was combined with either dimethyl phthalate (DMP) or dibutyl phthalate (DBP) to obtain initial mixtures with 55-148 ppm (w/w) sulfonate ester as tabulated below. The mixtures were then heated with vigorous stirring to 180° C., then cooled to 90° C. to simulate general esterification conditions. After such preparation, the resulting mixtures would have compositions similar to those observed in crude esterification product mixtures from typical commercial manufacturing processes. An aliquot of each mixture was taken at this point to represent the material prior to hydrolysis (i.e., time “0”). The hydrolysis medium was then added (30 ml water, aqueous 10% NaOH, or aqueous 10% Na2CO3) and the stirred mixture was maintained at 90° C. Aliquot samples were taken at the times specified in the table below. Each aliquot was extracted with diethyl ether, and the ether extracts washed with water, dried, and evaporated under vacuum to obtain the dry phthalate ester with residual sulfonate ester. The latter were then analyzed by gas chromatography to determine the amount of residual sulfonate ester. Initial Residual Sulfonate ester (ppm Sulfonate ester by weight) in Phthalate ester Phthalate Sulfonate Before Heating Hydrolysis after Hydrolysis Time (min.) Ester Ester (ppm w/w) Medium 0 15 30 60 120 180 DMP 200 ml MMS 55 Water 34 15 10 5.2 1.3 DMP 200 ml MTS 95 Water 101 98 93 93 83 DBP 200 ml BMS 55 Water 51 35 34 31 23 DBP 200 ml BTS 84 Water 81 78 76 76 72 DMP 100 ml MMS 136 Aq. NaOH 68 4 - <1 <1 - DMP 200 ml MTS 97 Aq. NaOH 70 64 53 40 24 DBP 200 ml BMS 48 Aq. NaOH 18 13 - 14 10 - DBP 200 ml BTS 84 Aq. NaOH 61 59 59 54 55 DMP 100 ml MMS 148Aq. Na2CO3 - 3 - <1 <1 - DBP 100 ml BMS 96Aq. Na2CO3 30 33 - 31 17 -
References:
US2006/30725,2006,A1 Location in patent:Page/Page column 6; 7
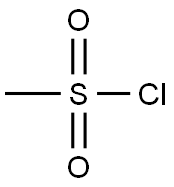
124-63-0
0 suppliers
$10.00/5g
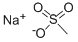
2386-57-4
233 suppliers
$13.00/25g
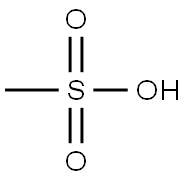
75-75-2
651 suppliers
$10.00/5g

1912-32-9
142 suppliers
$6.00/1g
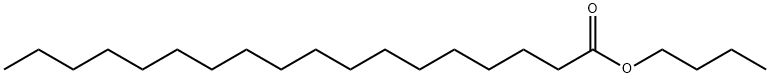
123-95-5
421 suppliers
$6.00/100g
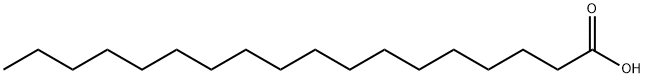
57-11-4
1255 suppliers
$6.00/100g

822-16-2
504 suppliers
$14.00/5g
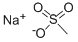
2386-57-4
233 suppliers
$13.00/25g
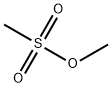
66-27-3
253 suppliers
$14.00/1g
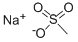
2386-57-4
233 suppliers
$13.00/25g