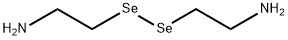
selenocystamine synthesis
- Product Name:selenocystamine
- CAS Number:2697-61-2
- Molecular formula:C4H12N2Se2
- Molecular Weight:246.07
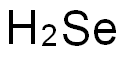
7782-49-2
264 suppliers
$27.00/25g
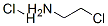
870-24-6
510 suppliers
$5.00/5g
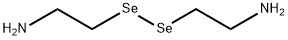
2697-61-2
25 suppliers
inquiry
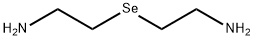
27974-50-1
0 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1:selenium with sodium tetrahydroborate;C12H18O12Pb3 in water at 20; for 1.33333 h;
Stage #2:2-chloroethanamine hydrochloride with sodium hydroxide in water at 20; for 17 h;
Steps:
1
To a 3 -neck flask fitted with an N2 gas inlet, an addition funnel, and a gas outlet leading to a trap containing a 5% Pb(OAc)2 solution, was added selenium metal (4.5 gm, 56.9 mmol), followed by 25 mL H2O. In a separate flask, NaBH4 (4.5 gm, 118.5 mmol) was taken up in 25 mL H2O and the solution was transferred to the addition funnel. The selenium metal slurry at room temperature was stirred at a moderate rate and the NaBH4 solution was slowly added drop wise at such a rate to keep the gas evolution and effervescence at a moderate pace. The solution changed from grey to brown-red and finally to a clear light yellow after complete addition (approximately 50 min.). After 10 minutes a second amount of selenium metal (4.5 gm, 56.9 mmol) was added in portions. The solution turned a dark red-brown color and was stirred for another 20 minutes at room temperature. A solution of 2-chloro-ethylamine hydrochloride (13.9 gm, 119.7 mmol) in 30 mL aqueous 20% sodium hydroxide was added to the reaction drop wise and stirring was continued at room temperature overnight (ca. 17 hours). The reaction was poured into a separatory funnel and extracted with CHCl3. The combined CHCl3 layers were dried over anhydrous MgSO4, filtered and concentrated to yield 9.8 gm of a light yellow oil, consisting of a mixture of 2,2'-diselenodiethanamine and 2,2'- selenodiethanamine. This material was dissolved in 200 mL of methanol/ethyl acetate (1 :1) and treated with anhydrous HCl in diethyl ether (IN solution). The solution was concentrated to yield a 10.1 gm of a light yellow solid. The solid was dissolved in methanol and ethyl acetate was slowly added until a precipitate started forming. Filtration, collection and drying of the solids produced 2.3 gm of 2,2'- diselenodiethanamine dihydrochloride as a light yellow powder.
References:
ANDOVER HEALTHCARE, INC. WO2007/109633, 2007, A2 Location in patent:Page/Page column 36
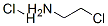
870-24-6
510 suppliers
$5.00/5g
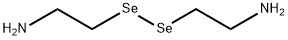
2697-61-2
25 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
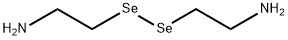
2697-61-2
25 suppliers
inquiry
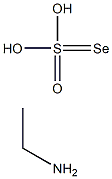
2697-60-1
0 suppliers
inquiry
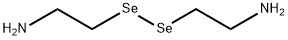
2697-61-2
25 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
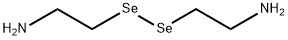
2697-61-2
25 suppliers
inquiry