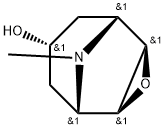
Scopine synthesis
- Product Name:Scopine
- CAS Number:498-45-3
- Molecular formula:C8H13NO2
- Molecular Weight:155.2
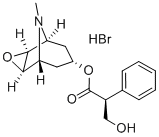
114-49-8
426 suppliers
$30.00/20mg

498-45-3
230 suppliers
$7.00/5mg
Yield:498-45-3 87%
Reaction Conditions:
with sodium tetrahydroborate in ethanol at 20;Cooling with ice;
Steps:
1 References:
WO2017/40778,2017,A1 Location in patent:Paragraph 00153-00157
Example 1: Synthesis of a Compound of Formula I A compound of Formula I was synthesized, from the compound of Formula XIII (Scopolamine [51-34-3]) ((2S)-(lR,2R,4S,5S)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7- yl-3-hydroxy-2-phenylpropanoate hydrobromide trihydrate) by the steps described below in Schemes 1 through 12. A first step is illustrated in Scheme 1 : Formula XIII Formula XII Inside a 10 liter four necked round bottom flask, sodium borohydride (172 g, 4558 mmol) was added portion wise over about 2 hours to a mechanically stirred suspension of a compound of Formula XIII (333 g, 760 mmol) in 3 liters of absolute ethanol in an ice bath. During this time gas formation occurred and the suspension was stirred while being warmed to ambient temperature overnight. While being heated, at approximately 10 °C, sudden additional gas formation and foaming occurred. The milky suspension was then concentrated to about half of its original volume (i.e. from about 3 L to 1.5 L) with additional precipitate observed, which yielded the batch. Meanwhile, 5 M HCl in isopropyl alcohol (IP A) (5318 mmol, 1.064 L) was diluted with 2 L of technical diethyl ether (Et20). The obtained hydrochloric acid (HCl) solution was then added drop wise to the ice-chilled batch, while being stirred. The white suspension was allowed to be mechanically stirred overnight to allow for full hydrolysis of the borate salts. The reaction mixture was filtered and the resulting solid was rinsed twice with 500 mL portions of Et20. The dried solid (which contained some Et20) was dissolved in a minimum amount of 10% aqueous potassium carbonate (K2C03) solution (-1.5 L) until just a clear solution was obtained. 200 mL of brine and -50 g solid NaCl was added to the solution. The aqueous phase was then thoroughly extracted with chloroform / methanol (MeOH) / [7N H3 in MeOH] (85: 14: 1). This procedure was performed 5 times with 1.0 L portions of this solvent mixture each. The combined organic extracts were dried (sodium sulphate (Na2S04)), filtered and the solvent was removed under reduced pressure to give 102.2 g (659 mmol) of a compound of Formula XII ((lR,2R,4S,5S)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-ol) as a slightly tan oil at 87% yield. 1H MR (CDC13) (Figure 1) showed structural agreement with the compound of Formula XII with minor amounts of impurities. 1H MR (400 MHz, Chloroform-
![3-Oxa-9-azatricyclo[3.3.1.02,4]nonane, 7-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-9-methyl-, (1α,2β,4β,5α,7β)- (9CI)](/CAS/20210305/GIF/182054-94-0.gif)
182054-94-0
0 suppliers
inquiry

498-45-3
230 suppliers
$7.00/5mg
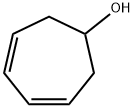
1121-63-7
1 suppliers
inquiry

498-45-3
230 suppliers
$7.00/5mg
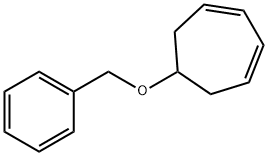
115522-58-2
0 suppliers
inquiry

498-45-3
230 suppliers
$7.00/5mg
132622-29-8
0 suppliers
inquiry

498-45-3
230 suppliers
$7.00/5mg