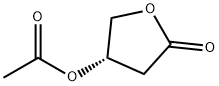
(S)-3-ACETOXY-GAMMA-BUTYROLACTONE synthesis
- Product Name:(S)-3-ACETOXY-GAMMA-BUTYROLACTONE
- CAS Number:191403-65-3
- Molecular formula:C6H8O4
- Molecular Weight:144.13
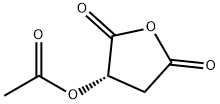
59025-03-5
89 suppliers
$20.10/2g

191403-65-3
18 suppliers
inquiry
Yield:191403-65-3 85%
Reaction Conditions:
Stage #1: (S)-acetoxysuccinic anhydridewith zinc(II) tetrahydroborate in tetrahydrofuran at 5 - 20; for 4 h;
Stage #2: with hydrogenchloride in water at 5; for 0.5 h;
Steps:
3 Example 3Preparation of (S)--Acetoxy-?-Butyrolactone Ic.
To Zn(BH4)2 (1.44g,15.8mmole) in 24ml THF, (S)--Acetoxy-?-succinic-anhydride 2 (5.0g, 31.6mmol) in 20ml THF was added below 5C over 1 hour and stirred at room temperature for 3 hours. The reaction mixture was cooled again below 5C, treated with 5ml of 6N HCI and stirred for half an hour. THF was removed under a rotary evaporator and oily water layer was extracted twice with 50ml of ethyl acetate. The combined ethyl acetate was dried over sodium sulfate, filtered and evaporated to yield 4.8g (85%) of yellow oil. IR (KBr): 1738 (C=0, acetoxy) and 1784 cm-1 (C=0, lactone).
References:
EP1398312,2004,A1 Location in patent:Page 6
