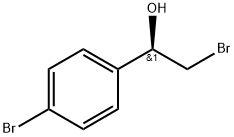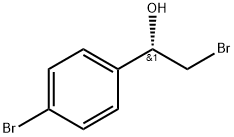
S)-2-broMo-1-(4-broMophenyl)ethanol synthesis
- Product Name:S)-2-broMo-1-(4-broMophenyl)ethanol
- CAS Number:100306-24-9
- Molecular formula:C8H8Br2O
- Molecular Weight:279.96
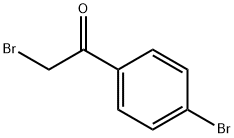
99-73-0
234 suppliers
$10.00/1g

100306-24-9
6 suppliers
inquiry
Yield:100306-24-9 100%
Reaction Conditions:
with dimethylsulfide borane complex;(S)-1-methyl-3,3-diphenyl-hexahydropyrrolo[1,2-c][1,3,2]oxazaborole in tetrahydrofuran at 0 - 20; for 12 h;
Steps:
18 ^-2-Bromo-l -(4-bromophenyl)ethanol (18.2)
To a stirred solution of (S)-l-methyl-3,3-diphenylhexahydropyrrolo[l,2- c][l,3,2]oxazaborole (0.5 g, 1.81 mmol) in THF (25 mL) was added BH3.DMS (10 M, 1.3 mL, 12.7 mmol) at 0 °C. The mixture was stirred for 0.5 h at 0 °C. To the reaction mixture was added a solution of 2-bromo-l-(4-bromophenyl)ethanone (5 g, 18.1 mmol) in THF (15 mL) dropwise at 0 °C. The mixture was stirred for 12 h at rt. To the mixture was added MeOH (the mixture was bubbled) dropwise. The MeOH was stopped to add after the mixture didn't bubble. The mixture was concentrated and purified by column chromatography on silica gel (PE/EA = 50/1 to 20/1 to 10/1 to 5/1) to give ()-2-bromo-l-(4-bromophenyl)ethanol (5 g, 100 % yield) as a white solid. MR (400 MHz, DMSO-d6) δ 7.55-7.52 (m, 2 H), 7.38-7.34 (m, 2 H), 5.89 (d, J = 4.9 Hz, 1 H), 4.82-4.78 (m, 1 H), 3.66 (dd, J= 10.2, 4.6 Hz, 1 H), 3.57 (dd, J= 10.2, 6.8 Hz, 1 H).
References:
WO2019/60693,2019,A1 Location in patent:Paragraph 00359-00360