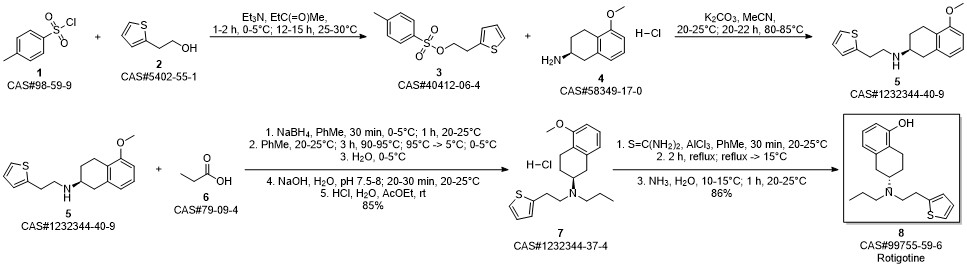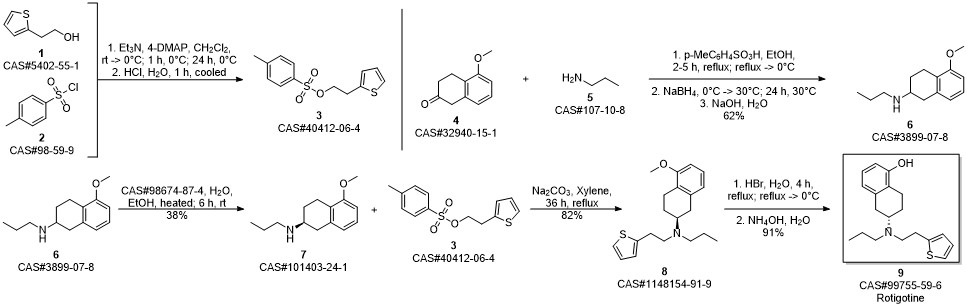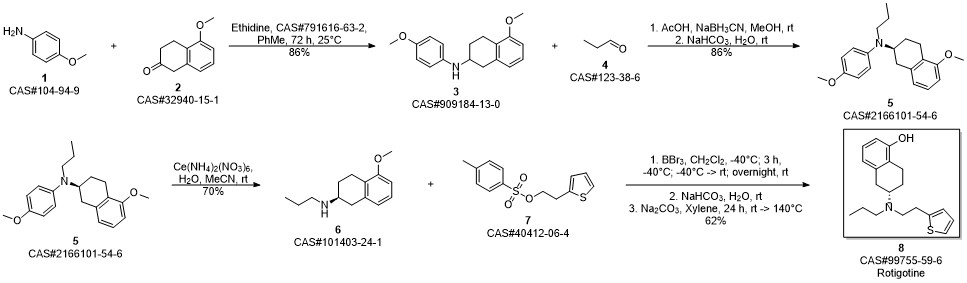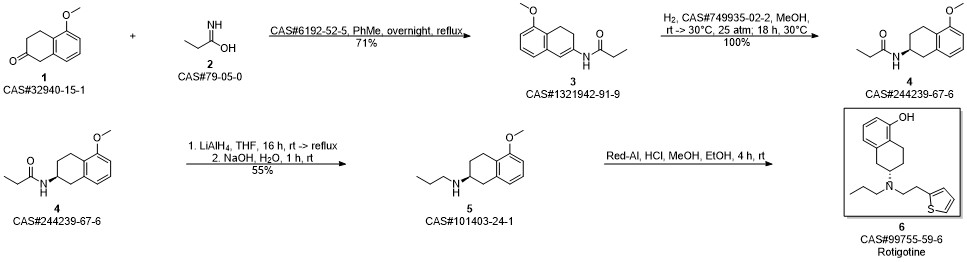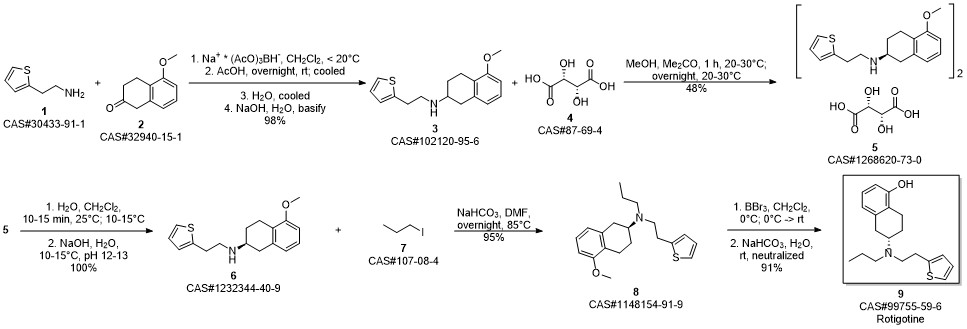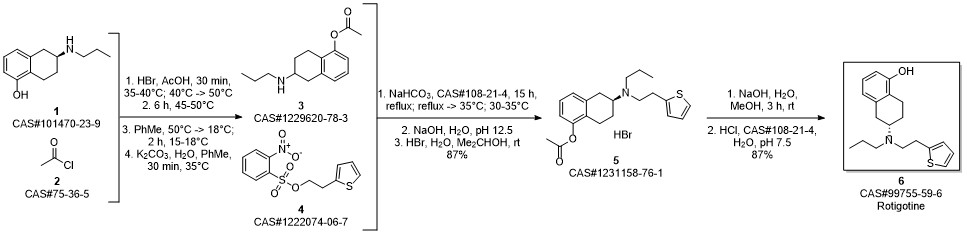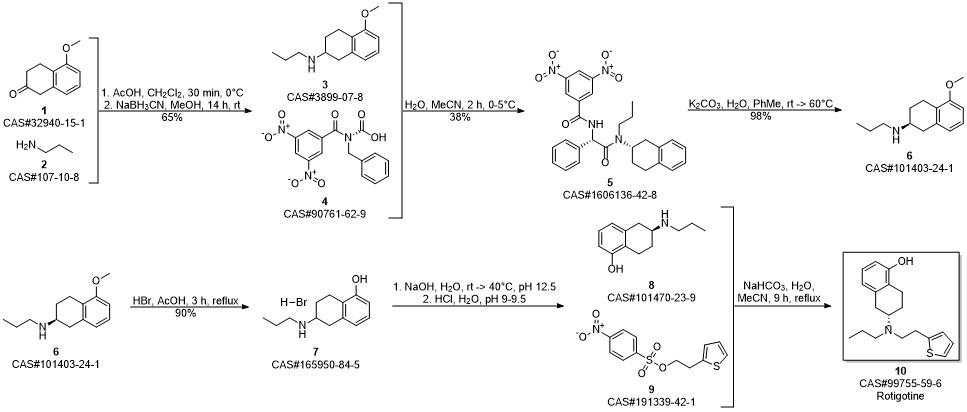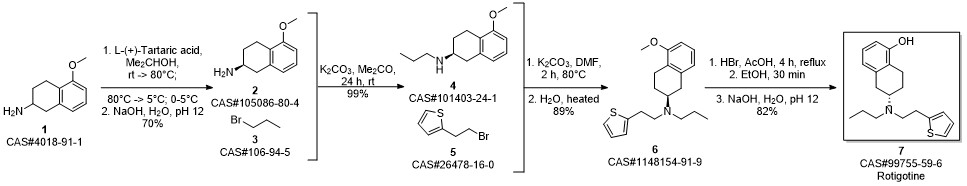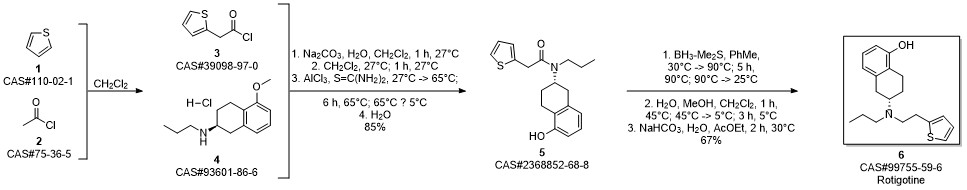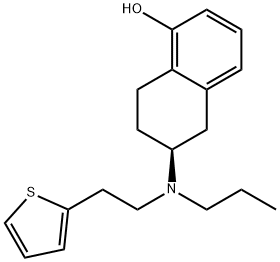
ROTIGOTINE synthesis
- Product Name:ROTIGOTINE
- CAS Number:99755-59-6
- Molecular formula:C19H25NOS
- Molecular Weight:315.47
Webster, Robert; Boyer, Alistair; Fleming, Matthew J.; Lautens, Mark. Practical Asymmetric Synthesis of Bioactive Aminotetralins from a Racemic Precursor Using a Regiodivergent Resolution. Organic Letters. Volume 12. Issue 23. Pages 5418-5421. 2010.
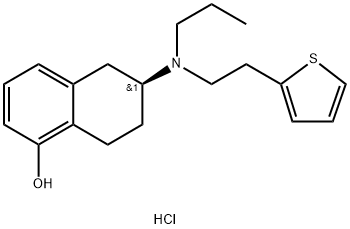
125572-93-2
161 suppliers
$35.00/5mg

99755-59-6
257 suppliers
$25.00/1mg
Yield:99755-59-6 98%
Reaction Conditions:
with sodium carbonate in dichloromethane;water
Steps:
13
Example 13 Dissociation of Rotigotine hydrochloride To a 10L four-necked reaction flask equipped with a mechanical agitator and a constant pressure dropping funnel, the above prepared 363g purified rotigotine hydrochloride and dichloromethane (5475mL) were added, and neutralized by dropwise addition of 5% aqueous sodium carbonate solution. The organic phase was separated out and washed with water once and concentrated to dryness to give 320g oil. Hexane (600mL) was added to the residue to precipitate a solid, which was filtered and dried to give rotigotine solid. Chemical Purity: 99.82%, Optical Purity: 100%, Yield: 98%. 1H-NMR(400MHz, CDCl3):δ 7.12(m, 1H, aromatic), 7.0(m, 1H, aromatic), 6.9(m, 1H, aromatic), 6.8(m, 1H, aromatic), 6.7(m, 1H, aromatic), 6.6(m, 1H, aromatic), 4.9(broad double, 1H, -OH), 2.8(m, 8H, -CH2-, -CH-), 2.6 (m, 3H, -CH2-), 2.10(m, 1H, -CH2-), 1.5(m, 3H, -CH2-), 0.9 (t, 3H, -CH3).
References:
2y-Chem, Ltd. EP2476676, 2012, A1 Location in patent:Page/Page column 9
![2-Thiopheneethanamine, N-propyl-N-[(2S)-1,2,3,4-tetrahydro-5-methoxy-2-naphthalenyl]-, hydrobromide (1:1)](/CAS/20211123/GIF/1222074-05-6.gif)
1222074-05-6
1 suppliers
inquiry

99755-59-6
257 suppliers
$25.00/1mg
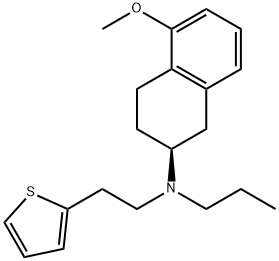
1148154-91-9
20 suppliers
inquiry

99755-59-6
257 suppliers
$25.00/1mg
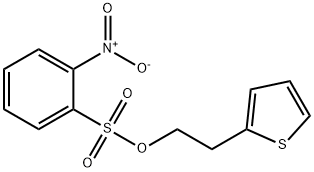
1222074-06-7
1 suppliers
inquiry
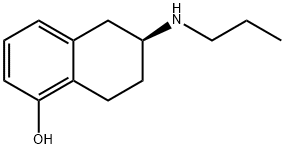
101470-23-9
36 suppliers
$85.00/10mg

99755-59-6
257 suppliers
$25.00/1mg
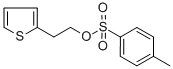
40412-06-4
250 suppliers
$6.00/5g

101470-23-9
36 suppliers
$85.00/10mg

99755-59-6
257 suppliers
$25.00/1mg