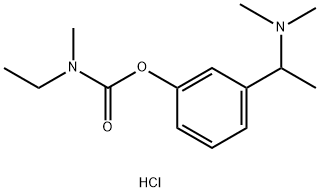
Rivastigmine hydrochloride synthesis
- Product Name:Rivastigmine hydrochloride
- CAS Number:105601-14-7
- Molecular formula:C14H23ClN2O2
- Molecular Weight:286.8
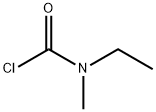
42252-34-6
286 suppliers
$7.00/1g
![3-(1-(Dimethylamino)ethyl]phenol](/CAS/GIF/105601-04-5.gif)
105601-04-5
249 suppliers
$6.00/1g

105601-14-7
3 suppliers
inquiry
Yield:105601-14-7 58%
Reaction Conditions:
Stage #1: N-ethyl-N-methylcarbamoyl chloride;(+/-)-3-<1-(Dimethylamino)ethyl>phenolwith sodium hydroxide in water;acetonitrile at 0 - 20; pH=11; for 24 h;O-carbamoylation;
Stage #2: with hydrogenchloride in diethyl ether;water at 20; for 1 h;
Steps:
1
[80] α-m-Hydroxyphenylethyldimethylamine (Formula 2, 50.0 g, 0.3 mol) was suspended in acetonitrile (250 ml), and then N-ethyl-N-methylcarbamoyl chloride (58.3 g, 0.48 mol) was added thereto. The reaction solution was cooled to 0°C. To the cooled solution was added sodium hydroxide (14.4 g, 0.36 mol). The resulting mixture was gradually allowed to rise to room temperature, and stirred at this temperature for 24 hours. The completion of the reaction was confirmed by HPLC. Thereafter, the reaction mixture was filtered to remove salts, and the obtained filtrate was concentrated. The pH of the concentrate was adjusted to 11 using water and NaOH solution, followed by extraction with ether and concentration. To the concentrate were added water and cone. HCl. The mixture was stirred at room temperature for one hour, and washed twice with ether. The obtained aqueous layer was concentrated, and re- crystallized from ethylacetate, yielding racemic rivastigmine hydrochloride (50.6 g, 58%). The racemic rivastigmine hydrochloride (20 g, 69 mmol) was dissolved in water (60 ml), and then NaOH (3.3 g, 1.2 eq.) was added thereto. The mixture was stirred at room temperature for one hour. The resulting mixture was extracted five times with ether, dried over anhydrous MgSO , and concentrated under reduced pressure. Di-O, O'4-p-toluoyl tartaric acid monohydrate (DTTA, 26.5 g, 69 mmol) and a solution of methanol/water (2/1) (180 ml) were added to the concentrate, dissolved under heating, and gradually allowed to cool to room temperature to obtain a precipitate. The precipitate was filtered, and recrystallized four times to afford rivastigmine DTTA salt (11.4 g, 26% (from the racemic rivastigmine hydrochloride)). The obtained rivastigmine DTTA salt (4.7 g, 7.4 mmol) was suspended in a IM NaOH solution (8 ml) EPO
References:
WO2006/68386,2006,A1 Location in patent:Page/Page column 8