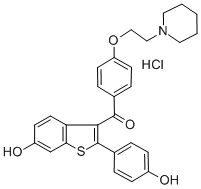
Raloxifene hydrochloride synthesis
- Product Name:Raloxifene hydrochloride
- CAS Number:82640-04-8
- Molecular formula:C28H28ClNO4S
- Molecular Weight:510.04
![6-METHOXY-2-(4-METHOXY PHENYL)-BENZO[B]THIEN-3-YL][4-[2-(1-[PIPERIDINYL)ETHOXY]PHENYL]METHANONE HYDROCHLORIDE](/CAS/GIF/84541-36-6.gif)
84541-36-6
14 suppliers
inquiry

82640-04-8
438 suppliers
$25.00/1g
Yield:82640-04-8 95%
Reaction Conditions:
Stage #1: [2-(4-methoxyphenyl)-6-methoxybenzo[b]thien-3-yl][4-[2-(1-piperidinyl)ethoxy]phenyl]methanone hydrochloridewith boron trichloride in dichloromethane at 2 - 20; for 52.25 h;
Stage #2: with methanol in dichloromethane at 12; for 1.08333 h;Product distribution / selectivity;Reflux;
Steps:
14.a
b) 1 -(2-{4-[6-Hydroxy-2-(4-hydroxy-phenyl)-benzo[b]thiophene-3-carbonyl]-phenoxy}- ethyl)-piperidinium chloride 0.37 methanol 0.51 methylene chloride:Boron trichloride in methylene chloride (1 M, 149 mL, 149 mmol) was cooled to 2 - 5 °C and 1 - (2-{4-[6-Methoxy-2-(4-methoxy-phenyl)-benzo[b]thio-phene-3-carbonyl]-phenoxy}-ethyl)- piperidinium chloride (20.0 g, 37.2 mmol) was added in portions over a period of 70 min with stirring. The mixture was stirred for 35 min at 3 - 4 °C and allowed to warm to ambient temperature within 90 min. Stirring was continued for 49 h after which the mixture was cooled to 12 °C. Methanol (60 mL) was then added slowly (approx. 20 min) and the resulting suspension was heated at reflux for 0.75 h. The solution was allowed to reach 20 °C and the solid was filtered off. The crystalline residue was with methanol (6 x 10 mL) and dried at 45°C under reduced pressure to give 1-(2-{4-[6-Methoxy-2-(4-methoxy-phenyl)-benzo[b]thiophene- 3-carbonyl]-phenoxy}-ethy.)-piperidinium chloride 0.37 methanol · 0.51 methylene chloride. Yield: 20.14 g (34.9 mmol, 95.0 %, calcd.). Purity: HPLC: 98.01 % (HPLC). Methanol: 2.1 % - by weight, corresponding to approx. 0.37 molar equivalents (GC). Methylene chloride: 7.7 % by weight, corresponding to approx. 0.51 molar equivalents (GC). Assay: 101 .7 % (AgN03). p.: 213 - 221 °C. DSC. Peak at 197.47 °C, Peak at 221 .81 °C, Peak at 334.13 °C. IR (KBr, cm"1): 3205, 2958, 2722, 2558, 1653 (vc=0), 1597, 1547, 1500, 1467, 1437, 1418, 1343, 1309, 1250, 1 1 66, 1065, 1037, 1020, 953, 938, 907, 840, 816, 808, 785, 738, 645, 635, 627, 594, 524. XRPD. XRD data correspond to those given for Example 14 item a).
References:
WO2011/47877,2011,A1 Location in patent:Page/Page column 67-68
![(4-(2-(PIPERIDIN-1-YL)ETHOXY)PHENYL)(6-METHOXY-2-(4-METHOXYPHENYL)BENZO[B]THIOPHEN-3-YL)METHANONE](/CAS/GIF/84541-38-8.gif)
84541-38-8
27 suppliers
$130.00/10mg

82640-04-8
438 suppliers
$25.00/1g
![2-[4-(Acetyloxy)phenyl]benzo[b]thiophene-6-ol acetate](/CAS2/GIF/84449-63-8.gif)
84449-63-8
23 suppliers
$498.67/5MG
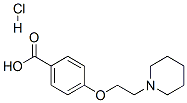
84449-80-9
196 suppliers
$10.00/1g

82640-04-8
438 suppliers
$25.00/1g
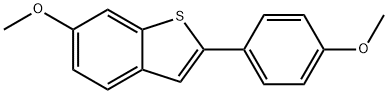
63675-74-1
286 suppliers
$5.00/250mg

84449-80-9
196 suppliers
$10.00/1g

82640-04-8
438 suppliers
$25.00/1g
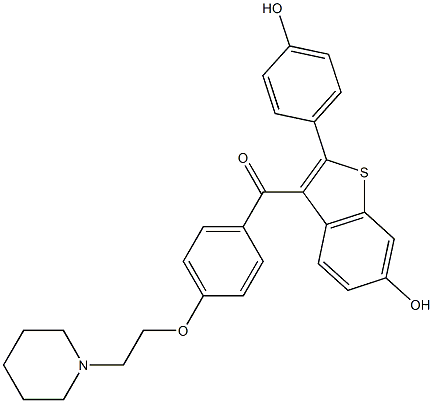
84449-85-4
14 suppliers
inquiry

82640-04-8
438 suppliers
$25.00/1g