rac-Naproxen-D3 Methyl Ester synthesis
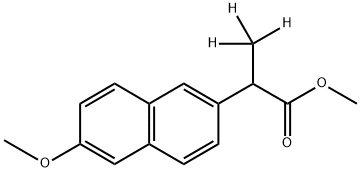
- Product Name:rac-Naproxen-D3 Methyl Ester
- CAS Number:1189511-76-9
- Molecular formula:C15H16O3
- Molecular Weight:244.29
Yield:-
Reaction Conditions:
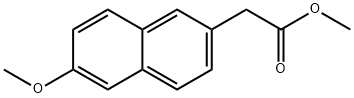
Stage #1: methyl 6-methoxy-2-naphthylacetatewith n-butyllithium;diisopropylamine in tetrahydrofuran at -78 - -40;
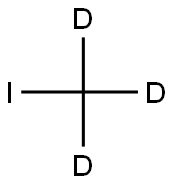
Stage #2: iodomethane-d3 in tetrahydrofuran at -78 - -40;
Steps:
5.5
Methyl 2-(6-methoxynaphthalen-2-yl)-Jj-propanoate:; At about -20 0C, 2.5M n-butyllithium in tetrahydrofuran was added to a solution of diisopropylamine (1.95 g, 19.3 mmol, 1.48 equiv.) in tetrahydrofuran (20 mL). The mixture was stirred at about 0 0C for about 30 minutes. After cooling the mixture to about -78 0C, a solution of methyl 2-(6-methoxynaphthalen-2-yl)acetate (3 g, 13 mmol, 1.00 equiv.) in tetrahydrofuran (30 mL) was then added. The mixture was stirred at about -40 0C for about 40 minutes. The mixture was cooled to about -78 0C, and then iodomethane (2.24 g, 15.4 mmol, 1.18 equiv.) was added. The mixture was stirred at about -40 0C for about 1 hour, and then a saturated aqueous solution of ammonium chloride (50 mL) was added. Standard extractive workup with ethyl acetate (2 x 50 mL) gave the title product as a brown oil (3.2 g; (crude)). LC-MS: m/z = 248 (MH)+. 1H NMR (300 MHz, CHCl3) δ: 7.69-7.74 (m, 3H), 7.51 (dd, / = 8.4, 1.8 Hz, IH), 7.14-7.18 (m, 2H), 3.94 (s, 3H), 3.87 (s, IH), 3.69 (s, 3H).
References:
WO2010/129505,2010,A2 Location in patent:Page/Page column 37-38