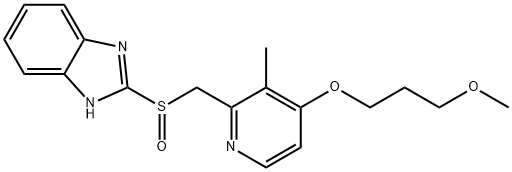
Rabeprazole synthesis
- Product Name:Rabeprazole
- CAS Number:117976-89-3
- Molecular formula:C18H21N3O3S
- Molecular Weight:359.44
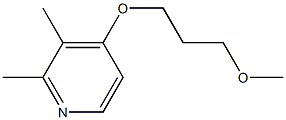
1080503-70-3
2 suppliers
inquiry
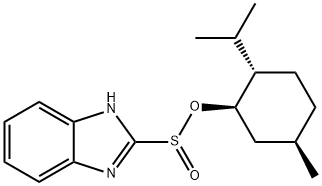
1080503-68-9
0 suppliers
inquiry

117976-89-3
239 suppliers
$15.00/1mg
Yield:117976-89-3 90%
Reaction Conditions:
Stage #1: 2,3-dimethyl-4-(3-methoxy-propoxy)pyridinewith n-butyllithium in tetrahydrofuran at -90 - -80;
Stage #2: (-)-menthyl 2-benzimidazolylsulphinate in tetrahydrofuran at -80 - -20;
Steps:
10
Example 10.- Preparation of rabeprazole, compound of general formula (11) wherein R2 is hydrogen, R4 is hydrogen, R5 is methyloxypropyloxy, and R6 is methyl; [Show Image] 4.26 g (21.8 mmol) of 2,3-dimethyl-4-(3-methoxy-propoxy)piridine were dissolved in 42 ml of anhydrous tetrahydrofuran at room temperature under inert atmosphere, and the solution was then cooled below - 90° C. 8.72 ml of a 2.5M solution of n-butyl lithium (21.8 mmol) were added dropwise and the temperature was maintained below -80° C. 2,3-dimethyl-4-(3-methoxy-propoxy)pyridine may be prepared in accordance with the process described in EP-A-0268956. After 30 minutes at this temperature, the mixture was slowly added to a solution of 2.0 g (6.24 mmol) of (-)-menthyl 2-benzimidazolylsulphinate, as obtained in Example 7, in 36 ml of tetrahydrofuran cooled below -80°C as well. Once the addition was completed, the resulting mixture was allowed to stand up to -20° C, then 100 ml of water were slowly added and it was allowed to reach room temperature. The reaction mixture was analyzed by HPLC, and conversion and yield were found to be 95% and 90% respectively.
References:
EP1992619,2008,A1 Location in patent:Page/Page column 16
![2-{[4-(3-Methoxypropoxy)-3-methylpyridine-2-yl]methylthio}-1H-benzimidazole](/CAS/GIF/117977-21-6.gif)
117977-21-6
341 suppliers
$8.00/5g

117976-89-3
239 suppliers
$15.00/1mg
924663-40-1
41 suppliers
$460.00/2mg

117976-89-3
239 suppliers
$15.00/1mg
![2-{[4-(3-Methoxypropoxy)-3-methylpyridine-2-yl]methylthio}-1H-benzimidazole](/CAS/GIF/117977-21-6.gif)
117977-21-6
341 suppliers
$8.00/5g

117976-89-3
239 suppliers
$15.00/1mg
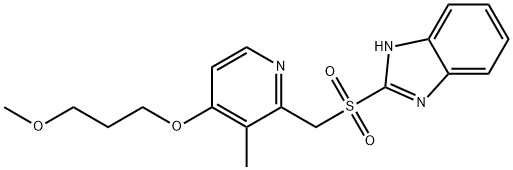
117976-47-3
91 suppliers
$40.00/5mg
408332-88-7
4 suppliers
inquiry
![2-{[4-(3-Methoxypropoxy)-3-methylpyridine-2-yl]methylthio}-1H-benzimidazole](/CAS/GIF/117977-21-6.gif)
117977-21-6
341 suppliers
$8.00/5g

117976-89-3
239 suppliers
$15.00/1mg