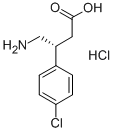
R(+)-Baclofen.Hydrochloride synthesis
- Product Name:R(+)-Baclofen.Hydrochloride
- CAS Number:63701-55-3
- Molecular formula:C10H13Cl2NO2
- Molecular Weight:250.12
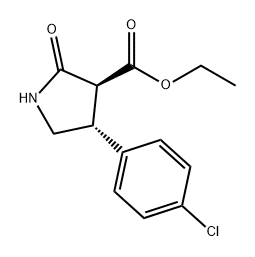
1201795-62-1
0 suppliers
inquiry

63701-55-3
71 suppliers
$40.00/10mg
Yield:99.4 % ee
Reaction Conditions:
Stage #1: (3S,4R)-ethyl 4-(4-chlorophenyl)-2-oxopyrrolidine-3-carboxylatewith hydrogenchloride;water in 1,2-dichloro-benzene at 100; for 24 h;
Stage #2: in water;toluene at 70 - 111;
Steps:
4
Synthesis Example 4; Synthesis of a hydrochloride salt of (R)-4-amino-3-(4-chlorophenyl)butanoic acidTo 7035 g of a solution containing 1618 g of ethyl (3S,4R)-4-(4-chlorophenyl)-2-oxopyrrolidine-3-carbonate, yielded in Synthesis Example 3, in 1,2-dichlorobenzene were added 2425 g of water and 3236 g of 35% hydrochloric acid, and then the solution was stirred at about 100° C. for 24 hours. The resultant reaction mixture was cooled, and then separated into two phases. To the water phase was added 3392 g of 1,2-dichlorobenzene to wash the phase, and then the solution was again separated into two phases. The resultant water phase was heated and refluxed, and then thereto was added 8417 g of toluene at 70 to 90° C. Until the temperature of the inside solution turned to 110° C., the solution was azeotropically dehydrated to distill off water. Next, toluene was distilled off until the inside temperature turned to 111° C. Thereto were added 143 g of water and 2494 g of acetonitrile, and the mixture was cooled. The solution was then stirred at about 20° C. for 1 hour. The mixture was filtrated, washed with a mixed liquid of 63 g of water and 2494 g of acetonitrile, and then dried. This way gave 1361 g of a hydrochloride salt of (R)-4-amino-3-(4-chlorophenyl)butanoic acid. The salt was analyzed under HPLC optical purity analysis conditions described below. As a result, the enantiomer excess thereof was 99.4%.
References:
US2011/152572,2011,A1 Location in patent:Page/Page column 5
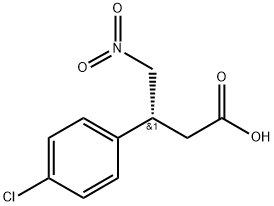
290366-79-9
2 suppliers
inquiry

63701-55-3
71 suppliers
$40.00/10mg
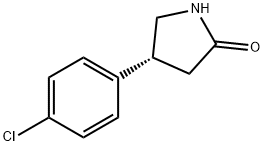
123632-35-9
12 suppliers
inquiry

63701-55-3
71 suppliers
$40.00/10mg
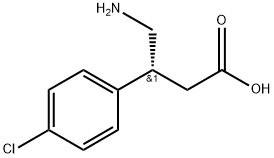
69308-37-8
192 suppliers
$15.00/5mg

63701-55-3
71 suppliers
$40.00/10mg
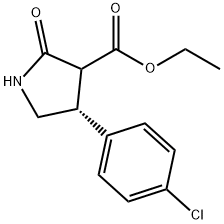
834917-63-4
1 suppliers
inquiry

63701-55-3
71 suppliers
$40.00/10mg
