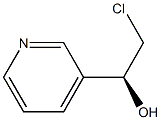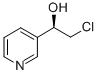
(R)-1-(Pyrid-3-yl)-2-chloroethanol synthesis
- Product Name:(R)-1-(Pyrid-3-yl)-2-chloroethanol
- CAS Number:173901-03-6
- Molecular formula:C7H8ClNO
- Molecular Weight:157.6
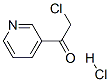
61889-48-3
77 suppliers
$18.00/100mg

173901-03-6
31 suppliers
inquiry
Yield:173901-03-6 82%
Reaction Conditions:
with triethylamine;(-)-diisopinocamphenylborane chloride in tetrahydrofuran at -25; for 72 h;
Steps:
1 (R)-2-Chloro-1-pyridin-3-ylethanol
To a solution of (-)-B-chlorodiisopinocampheylborane [(-)-DIP-Cl] (25 g, 77.9 mmol) in tetrahydrofuran (90 ml) are added with stirring 3-(2-chloroacetyl)pyridine hydrochloride (Can. J. Chem., vol. 61, p. 334 (1983)) (3.0 g, 15.6 mmol) and triethylamine (2.39 ml, 17.2 mmol) at -25°C, and the reaction mixture is stirred at -25°C for 3 days. To this mixture is added water (300 ml), and the mixture is warmed to room temperature. To the mixture is added ethyl acetate, and the organic phase is separated. The aqueous phase is neutralized with a saturated aqueous sodium hydrogen carbonate solution, and extracted 6 times with ethyl acetate. The combined organic phase is dried over sodium sulfate, and concentrated under reduced pressure to give yellow oil, which is purified by silica gel column chromatography (methanol/chloroform = 1/20) to give the title compound (R)-2-chloro-1-pyridin-3-ylethanol (2.02 g, yield: 82 %) as pale yellow oil. 1H-NMR (CDCl3) δ: 2.75 (1H, d, J=3.4Hz), 3.67 (1H, dd, J=8.5, 11.3Hz), 3.78 (1H, dd, J=3.5, 11.3Hz),, 4.96-5.00 (1H,m), 7.33 (1H, dd, J=4.9, 7.9Hz), 7.75-7.78 (1H, m), 8.59 (1H, dd, J=1.6, 4.8Hz), 8.64 (1H, d, J=2.2Hz).
References:
EP1514869,2005,A1 Location in patent:Page/Page column 20

55484-11-2
23 suppliers
inquiry

173901-03-6
31 suppliers
inquiry

350-03-8
666 suppliers
$10.00/5g

173901-03-6
31 suppliers
inquiry