(R)-1-(2,6-Dichloro-3-fluorophenyl)ethanol synthesis
- Product Name:(R)-1-(2,6-Dichloro-3-fluorophenyl)ethanol
- CAS Number:288385-87-5
- Molecular formula:C21H29N3O5
- Molecular Weight:403.47
264208-86-8
3 suppliers
inquiry

288385-87-5
3 suppliers
inquiry
Yield:288385-87-5 92%
Reaction Conditions:
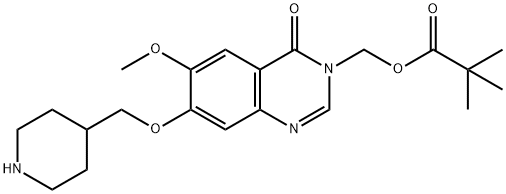
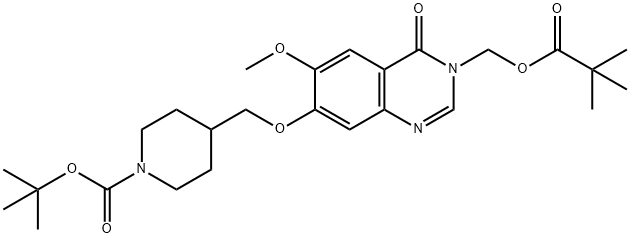
Stage #1: 7-(1-(tert-butoxycarbonyl)piperidin-4-ylmethoxy)-6-methoxy-3-((pivaloyloxy)methyl)-3,4-dihydroxyquinazolin-4-onewith trifluoroacetic acid in dichloromethane at 18 - 25; for 1 h;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate;
Steps:
2c Example 2c
A solution of 7-(1-(tert-butoxycarbonyl)piperidin-4-ylmethoxy)-6-methoxy-3-((pivaloyloxy)methyl)-3,4-dihydroquinazolin-4-one (2.32g, 4.6mmol) in methylene chloride (23ml) containing TFA (5ml) was stirred at ambient temperature for 1 hour. The volatiles were removed under vacuum. The residue was partitioned between ethyl acetate and sodium hydrogen carbonate. The organic solvent was removed under vacuum and the residue was filtered. The precipitate was washed with water, and dried under vacuum. The solid was azeotroped with toluene and dried under vacuum to give 6-methoxy-7-(piperidin-4-ylmethoxy)-3-((pivaloyloxy)methyl)-3,4-dihydroquinazolin-4-one (1.7g, 92%). MS - ESI: 404 [MH]+ 1H NMR Spectrum: (DMSOd6; CF3COOD) 1.15 (s, 9H), 1.45-1.6 (m, 2H), 1.95 (d, 2H), 2.1-2.25 (m, 1H), 2.95 (t, 2H), 3.35 (d, 2H), 3.95 (s, 3H), 4.1 (d, 2H), 5.95 (s, 2H), 7.23 (s, 1H), 7.54 (s, 1H), 8.45 (s, 1H)
References:
EP1244647,2006,B1 Location in patent:Page/Page column 23
123855-51-6
415 suppliers
$6.00/5g

288385-87-5
3 suppliers
inquiry
![7-Hydroxy-6-methoxy-3-[(pivaloyloxy)methyl]-3,4-dihydroquinazolin-4-one](/CAS2/GIF/193002-25-4.gif)
193002-25-4
4 suppliers
inquiry

288385-87-5
3 suppliers
inquiry