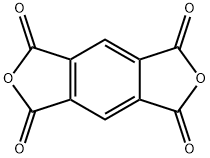
Pyromellitic Dianhydride synthesis
- Product Name:Pyromellitic Dianhydride
- CAS Number:89-32-7
- Molecular formula:C10H2O6
- Molecular Weight:218.12
C6H2(CH3)4 + 6 O2 → C6H2(C2O3)2 + 6 H2O
In the laboratory, it can be prepared by dehydration of pyromellitic acid using acetic anhydride.
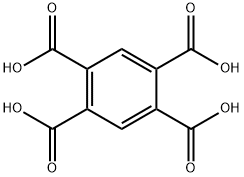
89-05-4
280 suppliers
$5.00/25g

89-32-7
383 suppliers
$10.00/5g
Yield:89-32-7 90%
Reaction Conditions:
2,6-bis[(2,2,6,6-tetramethylpiperidin-1-yl)methyl]phenylboronic acid in butryonitrile; for 12 h;Reflux;
Steps:
28
Examples 28 to 32Synthesis of a tetracarboxylic dianhydride used as a raw material for polyimide resin synthesis was investigated. In particular, in accordance with Examples 21 to 27, the dehydrative condensation reaction of a tetracarboxylic acid (2.5 mmol) shown in Table 5 was performed in butyronitrile (10 mL) in the presence of the boronic acid compound E (0.025 mmol, 1 percent by mole). After the reaction mixture was cooled to room temperature, 50 mL of pentane was added, and a precipitated solid was collected by filtration, so that a target tetracarboxylic dianhydride was obtained. In addition, in Example 32, instead of the isolated yield, the conversion yield was obtained by analysis using 1H NMR. The results are shown in Table 5. Each tetracarboxylic acid showed excellent reactivity, and a carboxylic dianhydride corresponding thereto was obtained in high yield (85% to 99%). Although the results obtained by reaction performed under no catalyst conditions were also shown in Table 5, the reaction hardly progressed under no catalyst conditions.
References:
US2011/319620,2011,A1 Location in patent:Page/Page column 10-11
18779-88-9
0 suppliers
inquiry

89-32-7
383 suppliers
$10.00/5g
95-93-2
225 suppliers
$21.00/25g

89-32-7
383 suppliers
$10.00/5g
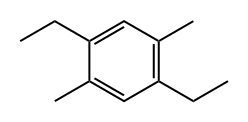
39144-22-4
0 suppliers
inquiry

89-32-7
383 suppliers
$10.00/5g
